Cerebral Vascular Stent Market Report
Published Date: 31 January 2026 | Report Code: cerebral-vascular-stent
Cerebral Vascular Stent Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cerebral Vascular Stent market, covering trends, market size, industry insights, and forecasts for the years 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
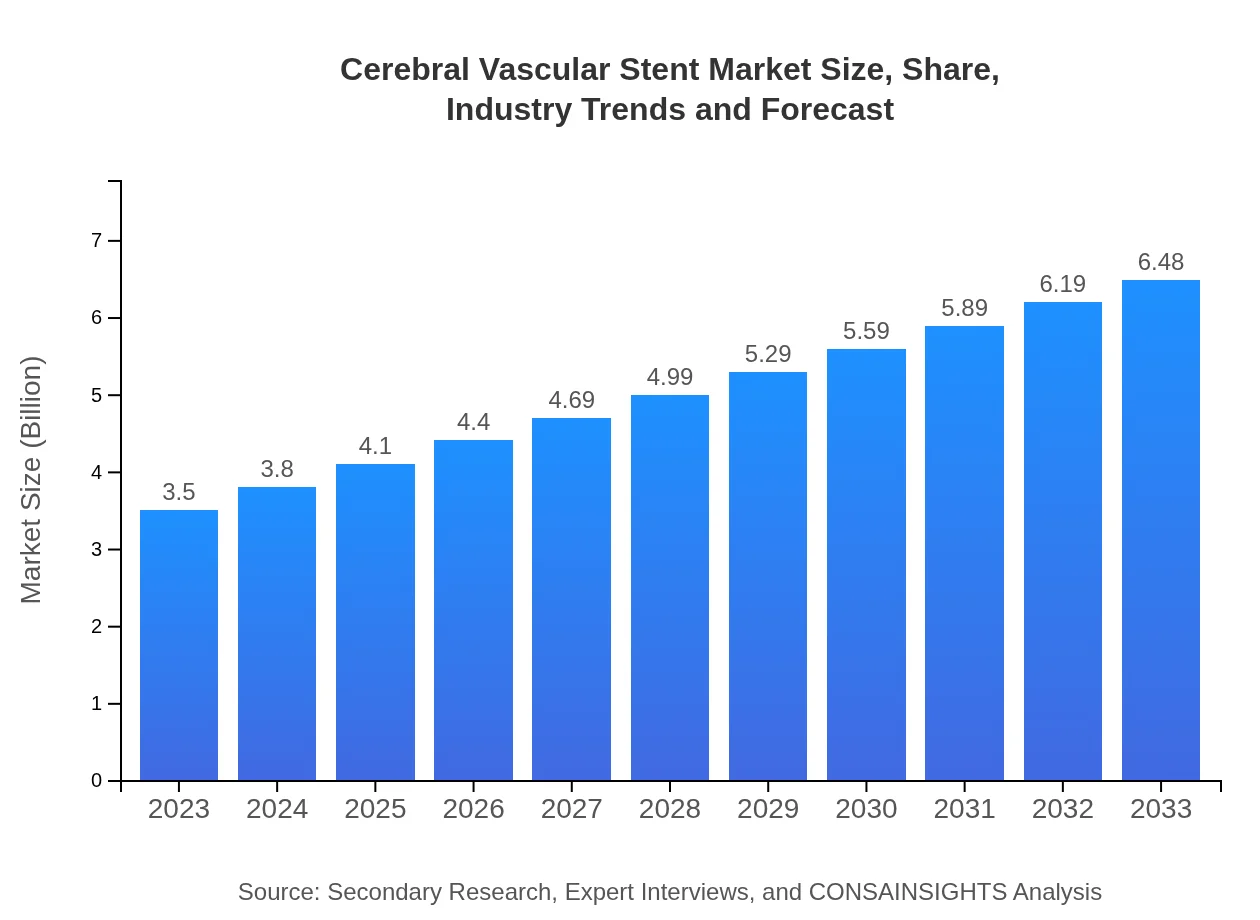
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $6.48 Billion |
| Top Companies | Medtronic , Boston Scientific, Abbott Laboratories, Stryker Corporation |
| Last Modified Date | 31 January 2026 |
Cerebral Vascular Stent Market Overview
Customize Cerebral Vascular Stent Market Report market research report
- ✔ Get in-depth analysis of Cerebral Vascular Stent market size, growth, and forecasts.
- ✔ Understand Cerebral Vascular Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cerebral Vascular Stent
What is the Market Size & CAGR of Cerebral Vascular Stent market in 2023?
Cerebral Vascular Stent Industry Analysis
Cerebral Vascular Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cerebral Vascular Stent Market Analysis Report by Region
Europe Cerebral Vascular Stent Market Report:
The European market stood at $1.02 billion in 2023 and projected to double to $1.89 billion by 2033. Factors contributing to this growth include a high prevalence of neurovascular diseases, an aging population, and significant investments in research and healthcare services.Asia Pacific Cerebral Vascular Stent Market Report:
In the Asia Pacific region, the market was valued at approximately $0.64 billion in 2023 and is projected to grow to $1.19 billion by 2033. This growth is fueled by increasing healthcare expenditure and rising awareness about stroke prevention services. Technological advancements and government initiatives supporting healthcare infrastructure are also anticipated to drive the regional market.North America Cerebral Vascular Stent Market Report:
North America dominates the Cerebral Vascular Stent market, with an estimated value of $1.33 billion in 2023, expected to rise to $2.46 billion by 2033. The robust growth can be attributed to high healthcare spending, advanced medical technology adoption, and stringent regulations ensuring high-quality healthcare delivery.South America Cerebral Vascular Stent Market Report:
The South American market for cerebral vascular stents was valued at about $0.24 billion in 2023, with projections of reaching $0.44 billion by 2033. The growth in this region is largely influenced by improvements in healthcare access and a growing number of hospitals adopting advanced medical technologies.Middle East & Africa Cerebral Vascular Stent Market Report:
In the Middle East and Africa, the market size was valued at approximately $0.27 billion in 2023, with forecasts predicting an increase to $0.49 billion by 2033. This growth is driven by improved healthcare infrastructure, initiatives aimed at enhancing healthcare delivery, and increasing demand for innovative treatment solutions.Tell us your focus area and get a customized research report.
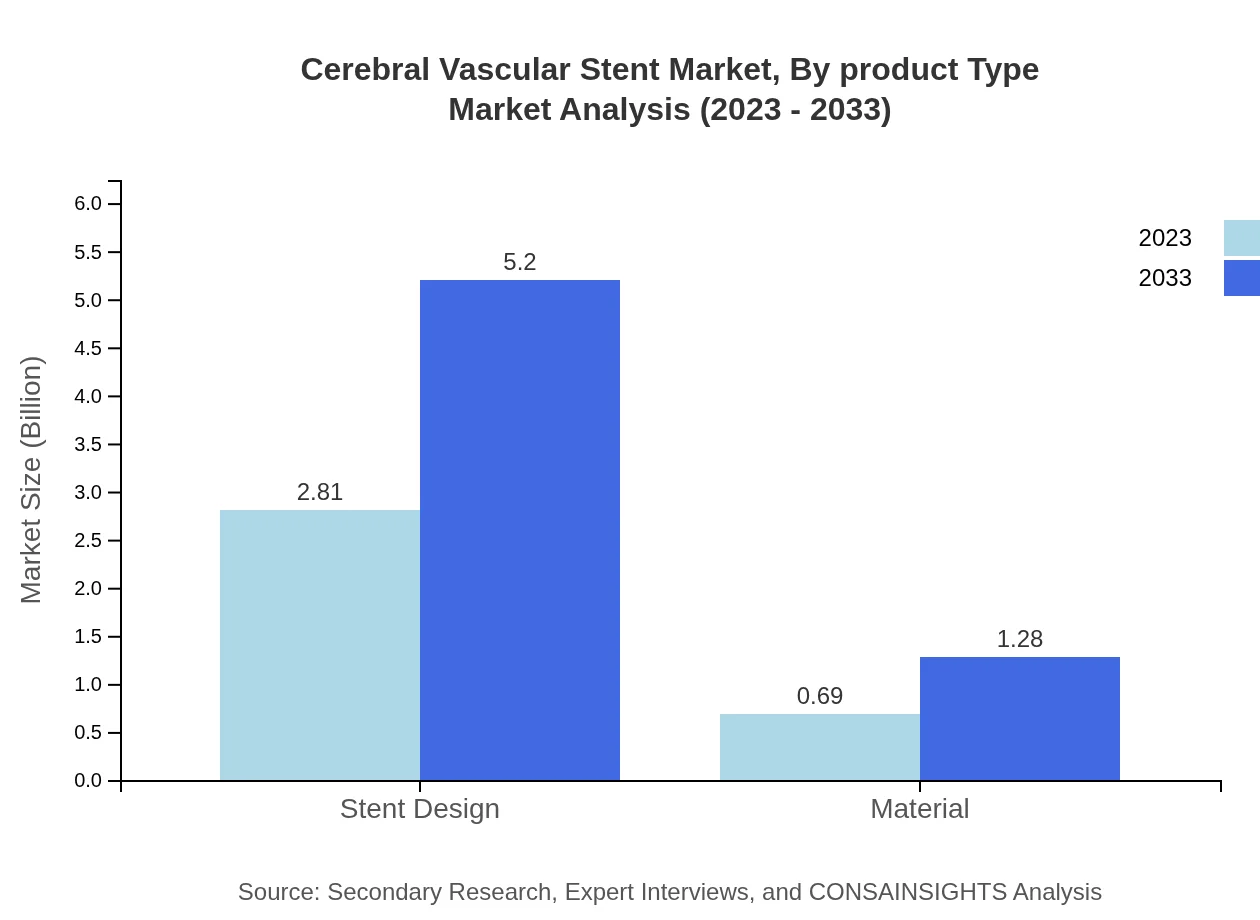
Cerebral Vascular Stent Market Analysis By Product Type
The product type segment comprises two main categories: drug-coated stents and bare-metal stents. In 2023, the market value for drug-coated stents is estimated at $2.81 billion with an expected increase to $5.20 billion by 2033, maintaining an 80.2% market share. The bare-metal segment, while smaller, is significant, providing essential treatment options in circumstances where drug-eluting technology may not be suitable.
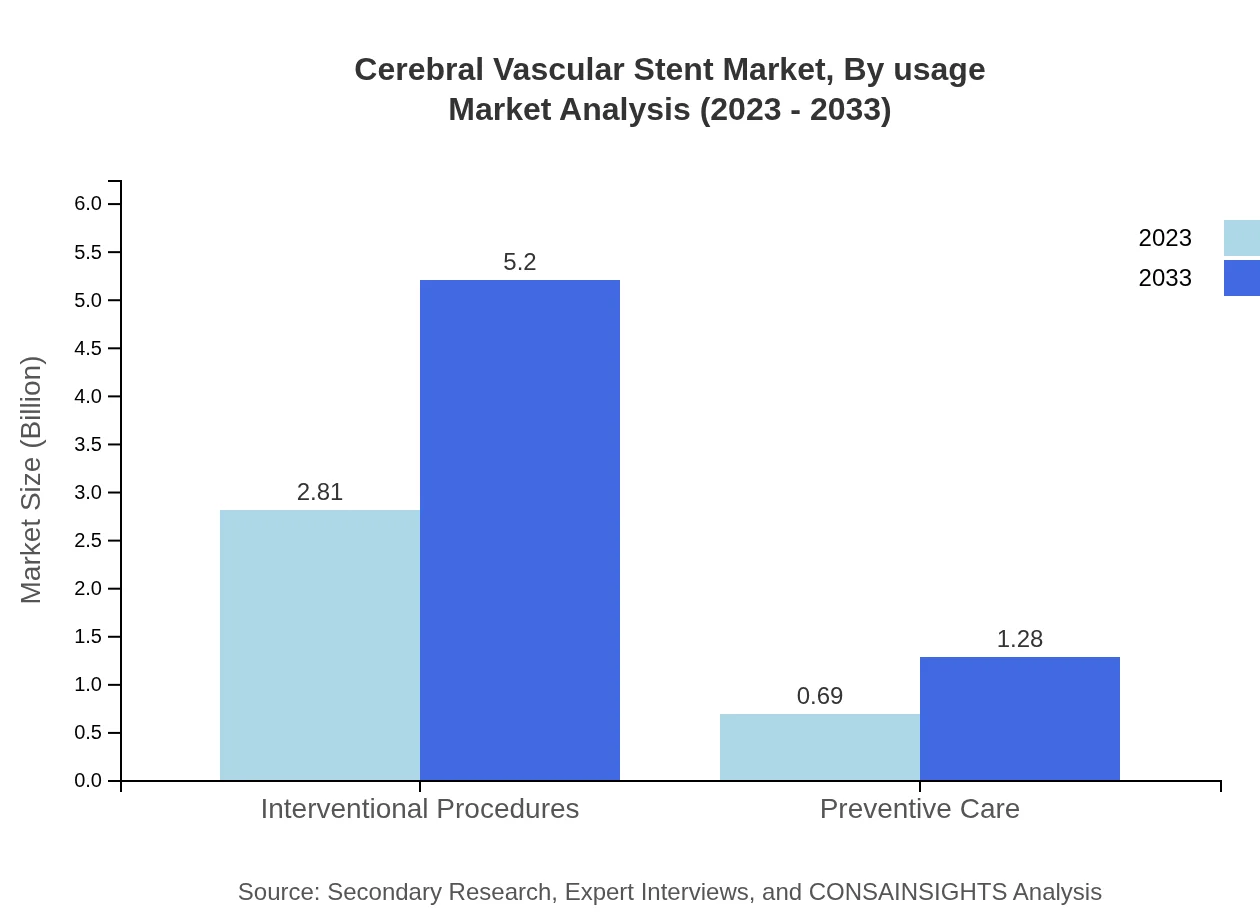
Cerebral Vascular Stent Market Analysis By Usage
The usage segment illustrates substantial growth in interventional procedures, which is poised to drive the market from $2.81 billion in 2023 to $5.20 billion by 2033. This area holds an impressive 80.2% market share. Preventive care is also gaining traction, expected to rise from $0.69 billion to $1.28 billion during the same period.
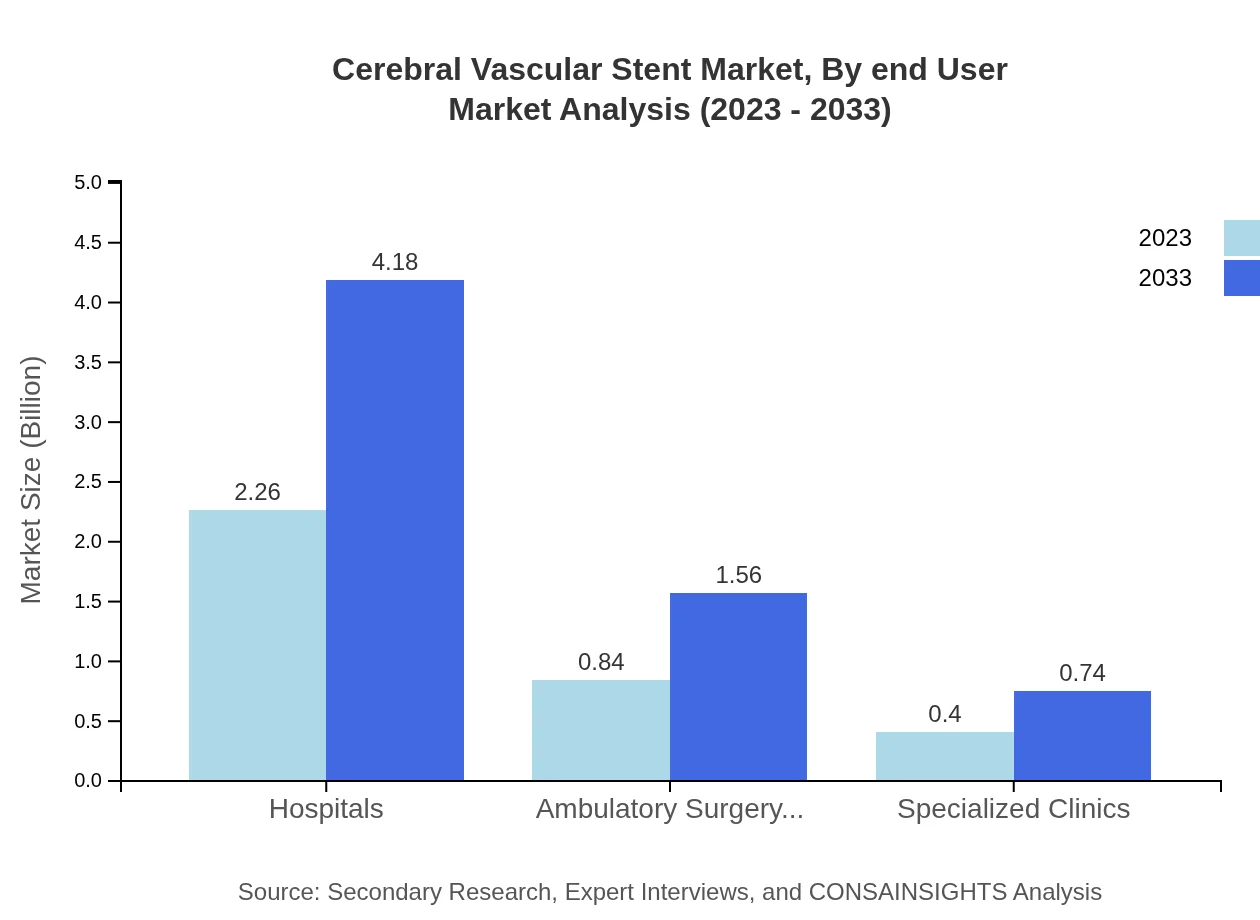
Cerebral Vascular Stent Market Analysis By End User
Hospitals remain the predominant end-user, with a market size of $2.26 billion in 2023, projected to grow to $4.18 billion by 2033, representing 64.52% of the market share. Ambulatory surgery centers and specialized clinics play crucial roles, particularly in enhancing patient access to advanced healthcare services.
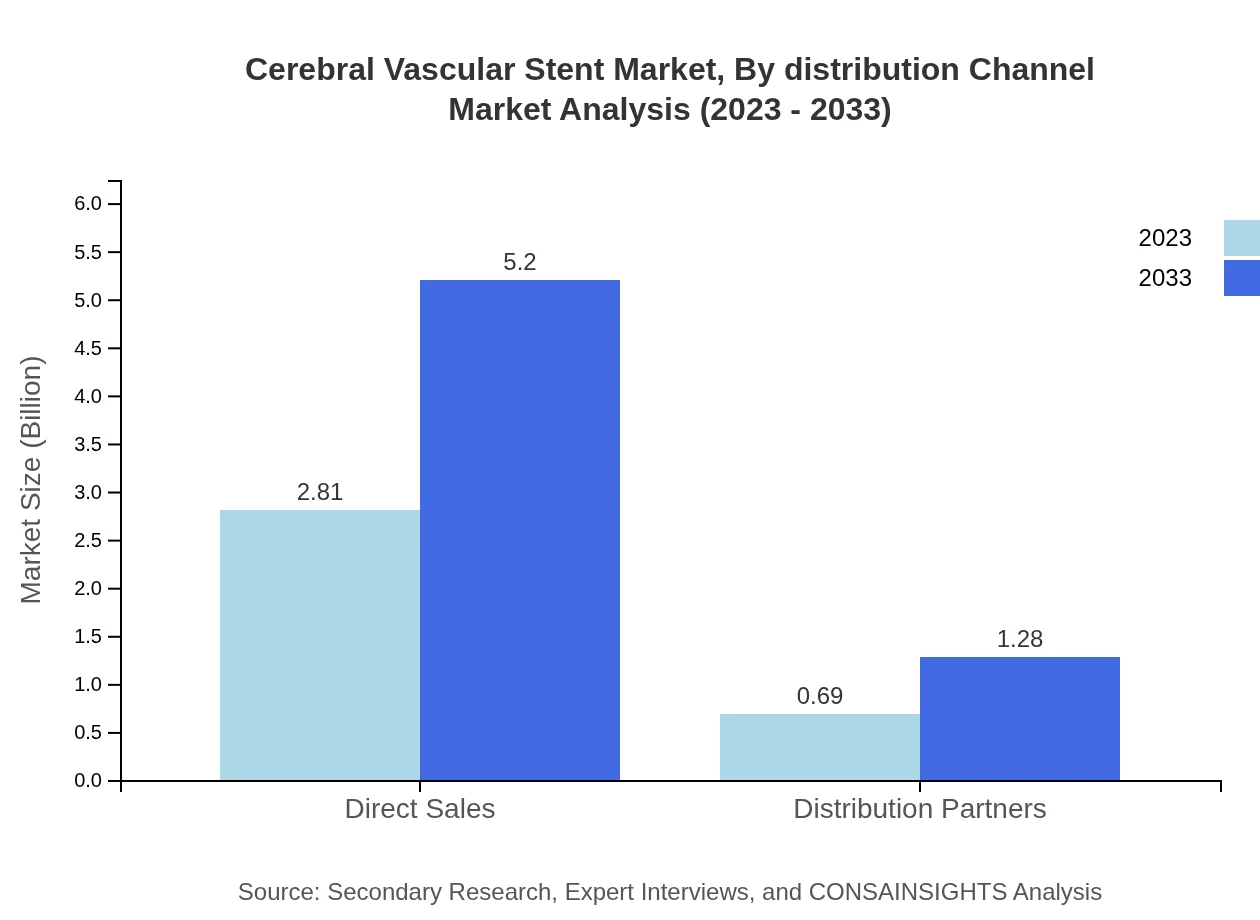
Cerebral Vascular Stent Market Analysis By Distribution Channel
The distribution channel segment indicates direct sales grossing $2.81 billion in 2023, with projections to $5.20 billion by 2033, maintaining an 80.2% market share. Conversely, distribution partnerships are expected to develop further, growing from $0.69 billion to $1.28 billion within the forecast period.
Cerebral Vascular Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cerebral Vascular Stent Industry
Medtronic :
A global leader in medical technology, Medtronic offers innovative solutions in vascular therapy, including advanced cerebral vascular stents.Boston Scientific:
Known for their commitment to advancing healthcare, Boston Scientific develops high-quality stents designed for optimal performance in neurovascular applications.Abbott Laboratories:
Recognized for pioneering cardiovascular medical devices, Abbott designs and manufactures cutting-edge cerebral stenting products aimed at improving patient outcomes.Stryker Corporation:
Stryker specializes in providing various medical devices, including stents, focusing on enhancing procedural outcomes through innovative designs.We're grateful to work with incredible clients.









FAQs
What is the market size of cerebral vascular stent?
The cerebral vascular stent market size is projected to reach approximately $3.5 billion by 2033, growing at a CAGR of 6.2%. This growth reflects increasing demand for advanced medical devices in neurovascular interventions.
What are the key market players or companies in the cerebral vascular stent industry?
Key players in the cerebral vascular stent industry include leading medical device companies focused on neurovascular technologies, enhancing treatment efficacy and patient care. Their innovations significantly influence market growth and competitive dynamics.
What are the primary factors driving the growth in the cerebral vascular stent industry?
Growth in the cerebral vascular stent market is driven by rising incidence of cerebral vascular diseases, advancements in stent design and material technology, and increasing healthcare expenditure focusing on sophisticated treatment options for patients.
Which region is the fastest Growing in the cerebral vascular stent market?
The North America region exhibits the fastest growth in the cerebral vascular stent market, projected to increase from $1.33 billion in 2023 to $2.46 billion by 2033, driven by technological advancements and extensive healthcare infrastructure.
Does ConsaInsights provide customized market report data for the cerebral vascular stent industry?
Yes, ConsaInsights offers customized market report data tailored to specific requirements within the cerebral vascular stent industry. This flexibility allows businesses to gain crucial insights tailored to their strategic needs.
What deliverables can I expect from this cerebral vascular stent market research project?
From this market research project, expect comprehensive reports including market size data, regional analysis, competitive landscape overviews, and trend insights tailored specifically to the cerebral vascular stent industry.
What are the market trends of cerebral vascular stent?
Current trends in the cerebral vascular stent market include a shift towards minimally invasive techniques, innovative stent designs, and increasing patient awareness regarding neurovascular health, all contributing to positive market growth.