Cerebrospinal Fluid Csf Management Devices Market Report
Published Date: 31 January 2026 | Report Code: cerebrospinal-fluid-csf-management-devices
Cerebrospinal Fluid Csf Management Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cerebrospinal Fluid (CSF) Management Devices market, including detailed insights into market trends, size, growth forecasts, and regional dynamics from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
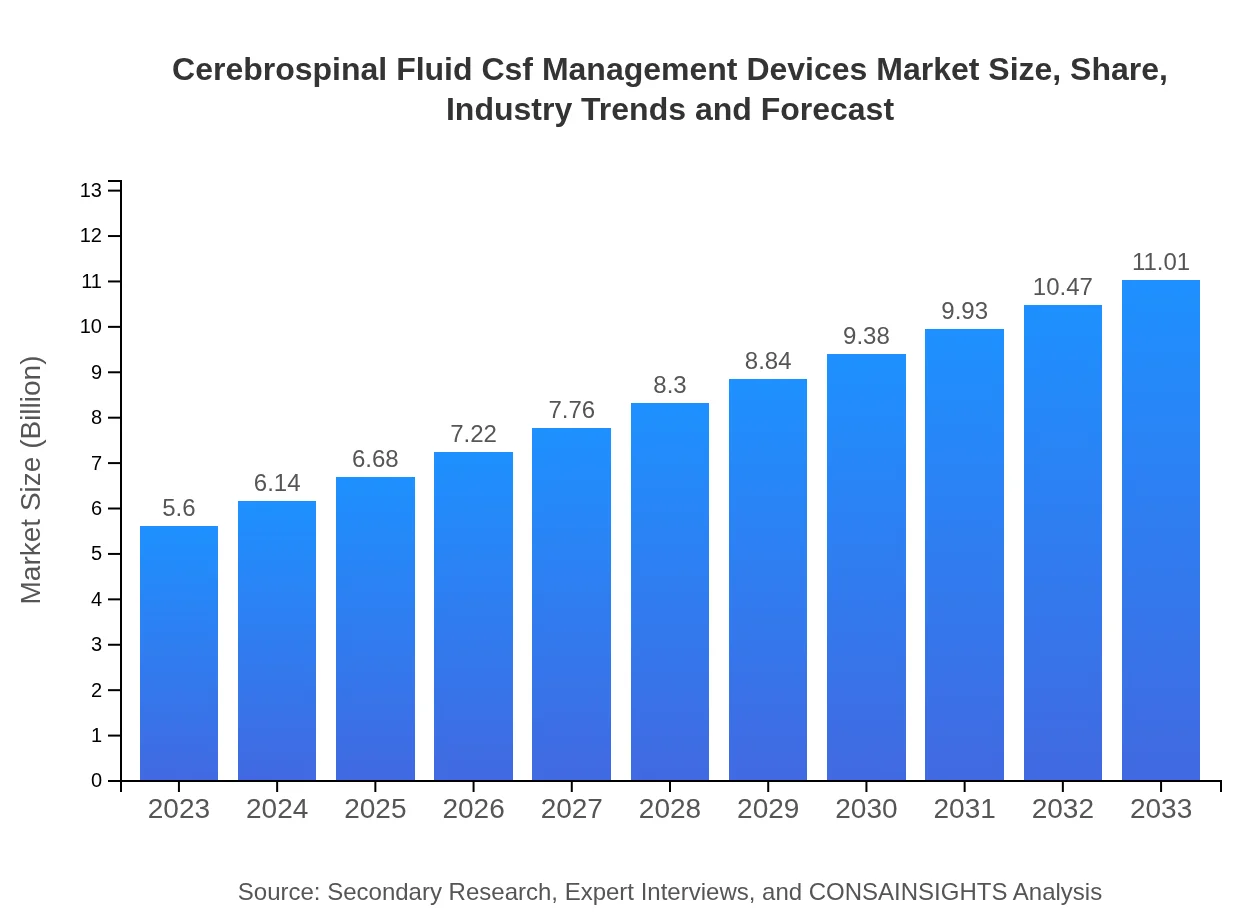
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $11.01 Billion |
| Top Companies | Medtronic , Abbott Laboratories, B. Braun Melsungen AG, Codman Neuro, Stryker |
| Last Modified Date | 31 January 2026 |
Cerebrospinal Fluid Csf Management Devices Market Overview
Customize Cerebrospinal Fluid Csf Management Devices Market Report market research report
- ✔ Get in-depth analysis of Cerebrospinal Fluid Csf Management Devices market size, growth, and forecasts.
- ✔ Understand Cerebrospinal Fluid Csf Management Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cerebrospinal Fluid Csf Management Devices
What is the Market Size & CAGR of Cerebrospinal Fluid Csf Management Devices market in 2023?
Cerebrospinal Fluid Csf Management Devices Industry Analysis
Cerebrospinal Fluid Csf Management Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
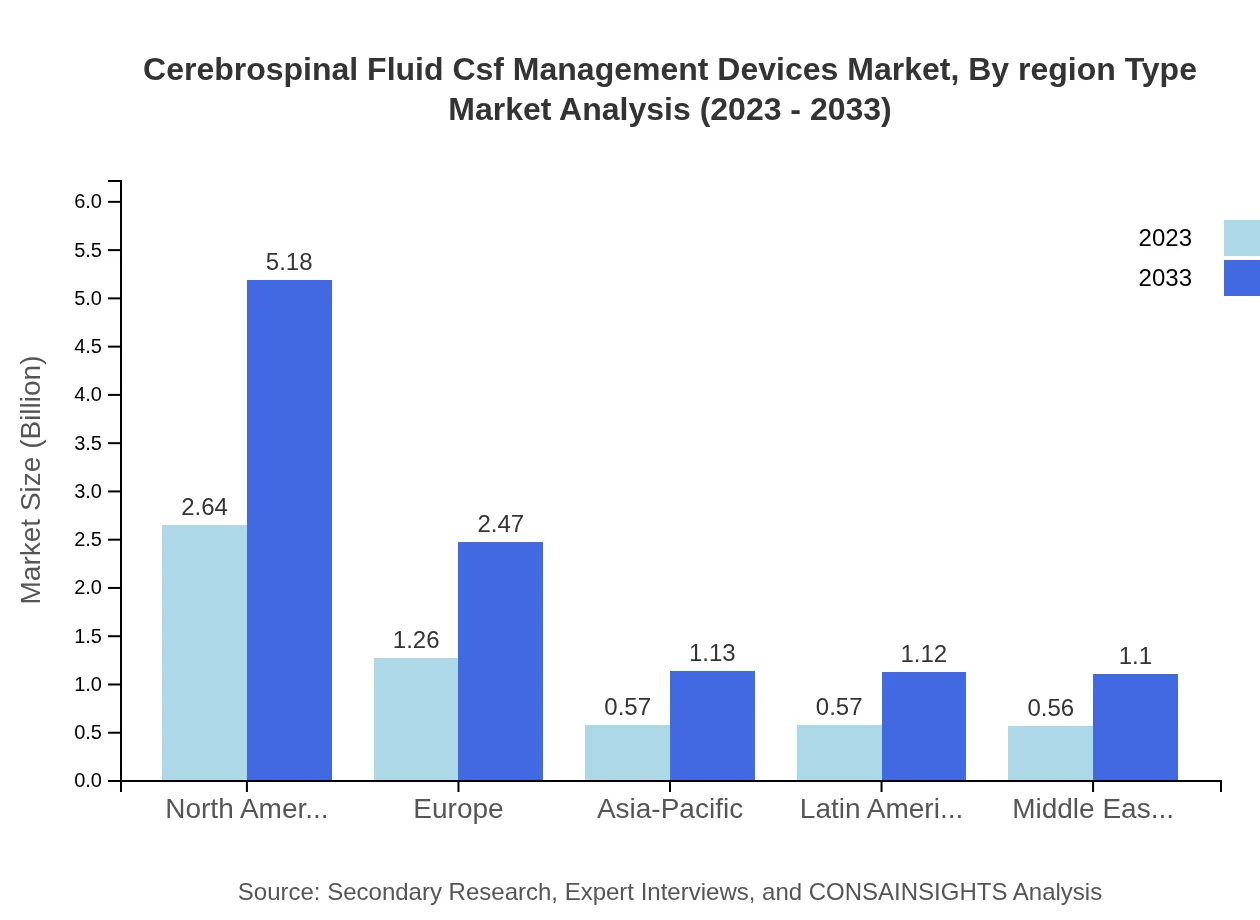
Cerebrospinal Fluid Csf Management Devices Market Analysis Report by Region
Europe Cerebrospinal Fluid Csf Management Devices Market Report:
Europe's market is forecasted to grow from USD 1.42 billion in 2023 to USD 2.80 billion by 2033, supported by increasing investments in neurological research and a robust healthcare system.Asia Pacific Cerebrospinal Fluid Csf Management Devices Market Report:
The Asia-Pacific region is anticipated to exhibit considerable growth in the CSF management devices market, with an increase from USD 1.07 billion in 2023 to USD 2.09 billion in 2033, driven by increasing healthcare expenditure and technological advancements in medical devices.North America Cerebrospinal Fluid Csf Management Devices Market Report:
North America leads the market with a size projected to double from USD 1.96 billion in 2023 to USD 3.86 billion in 2033. This growth is largely due to the high prevalence of neurological disorders and strong adoption of advanced medical technologies.South America Cerebrospinal Fluid Csf Management Devices Market Report:
The South American market is also expected to grow, reaching USD 0.80 billion in 2033 from USD 0.40 billion in 2023. Enhanced medical infrastructure and rising awareness of neurological conditions are key growth factors.Middle East & Africa Cerebrospinal Fluid Csf Management Devices Market Report:
In the Middle East and Africa, the market is expected to grow from USD 0.74 billion in 2023 to USD 1.46 billion in 2033, with infrastructure development and improved access to medical care contributing to this growth.Tell us your focus area and get a customized research report.
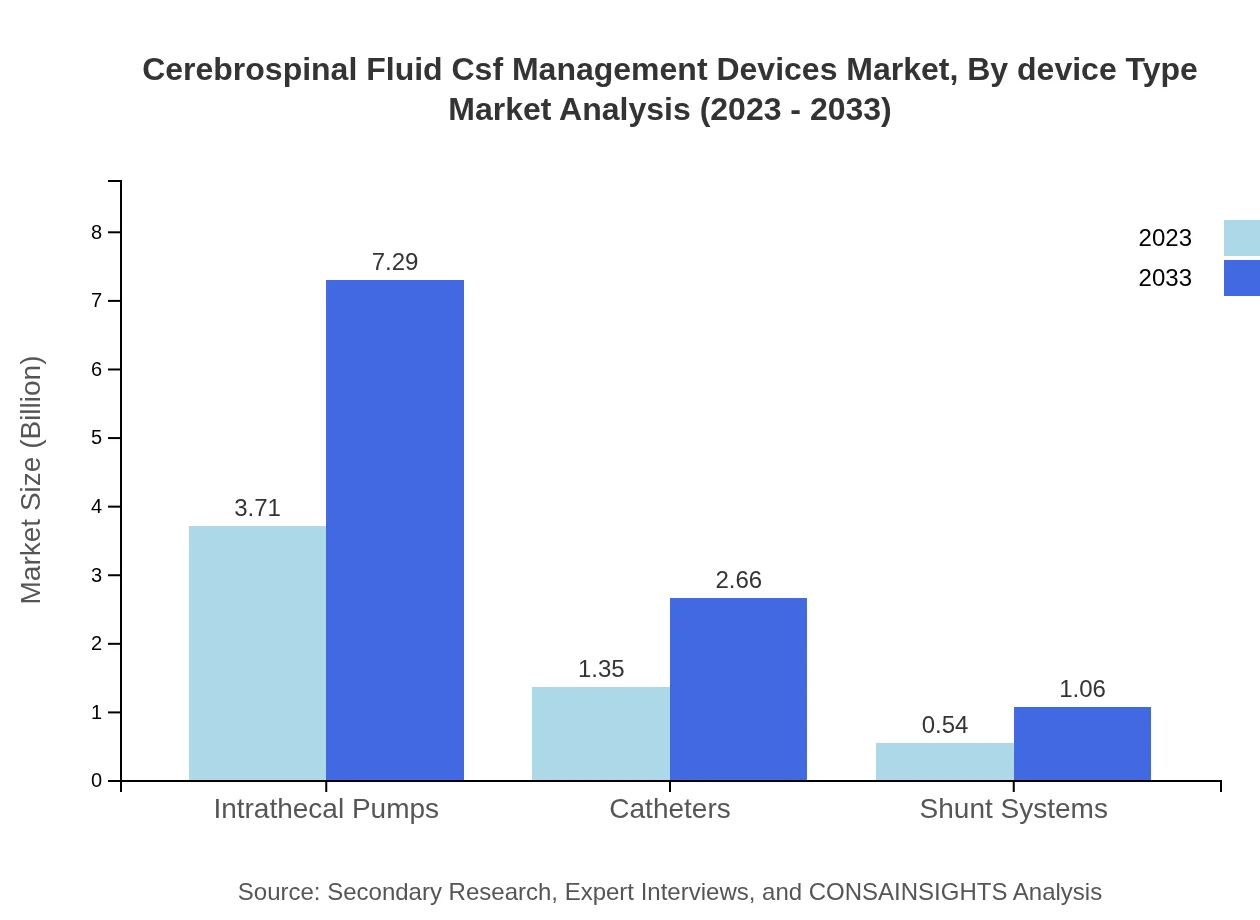
Cerebrospinal Fluid Csf Management Devices Market Analysis By Device Type
The CSF management devices market by device type includes significant segments such as Intrathecal Pumps, which dominate the market with a size forecasted to expand from USD 3.71 billion in 2023 to USD 7.29 billion by 2033, accounting for 66.24% market share. Catheters also hold a significant position, projected to grow from USD 1.35 billion to USD 2.66 billion, with a 24.13% market share. Shunt Systems, while smaller in size, will see growth from USD 0.54 billion to USD 1.06 billion.
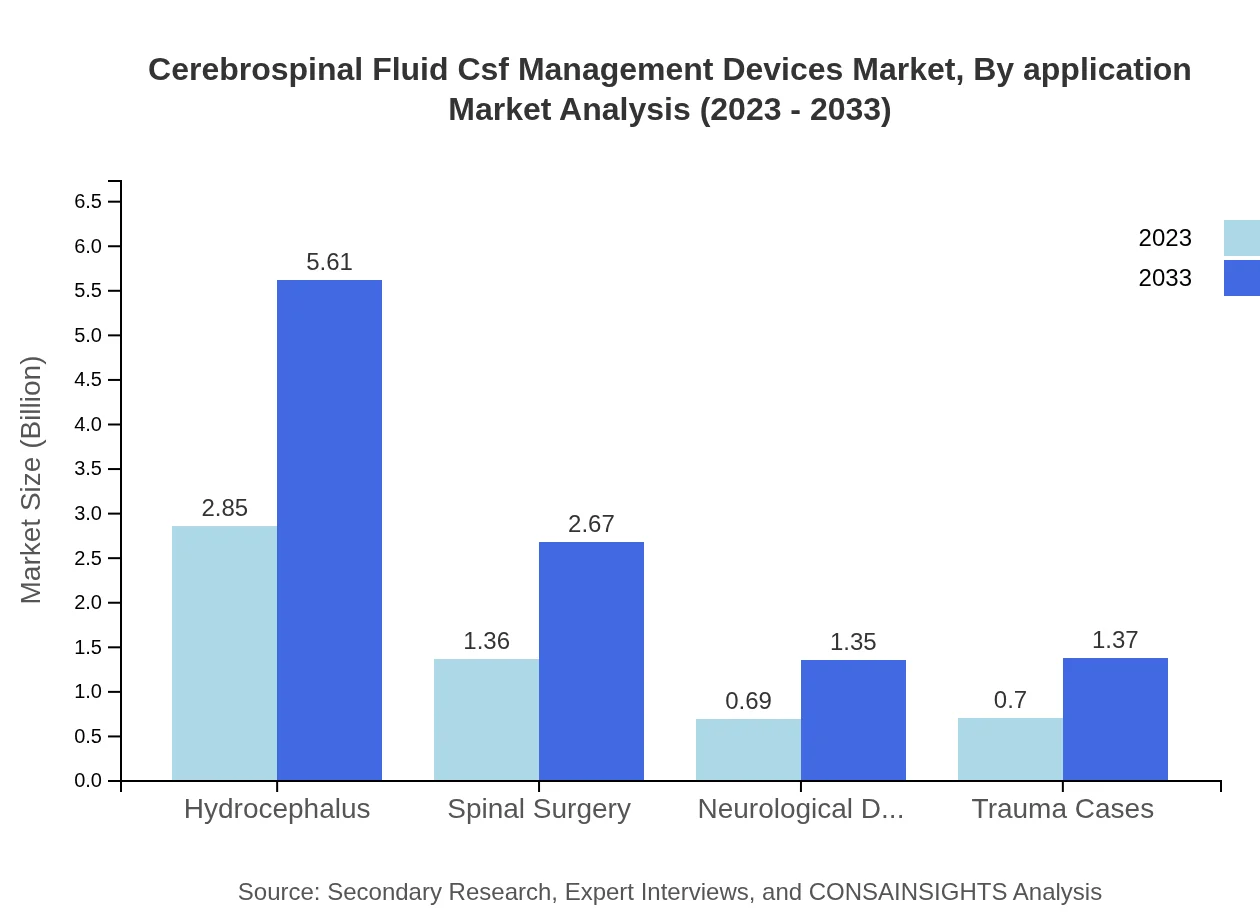
Cerebrospinal Fluid Csf Management Devices Market Analysis By Application
The application segments of CSF management devices include Hydrocephalus, which leads the market with a significant growth trajectory, expanding from USD 2.85 billion in 2023 to USD 5.61 billion by 2033. Other significant applications include spinal surgery and management of neurological disorders, each with data indicating steady growth over the forecasted period.
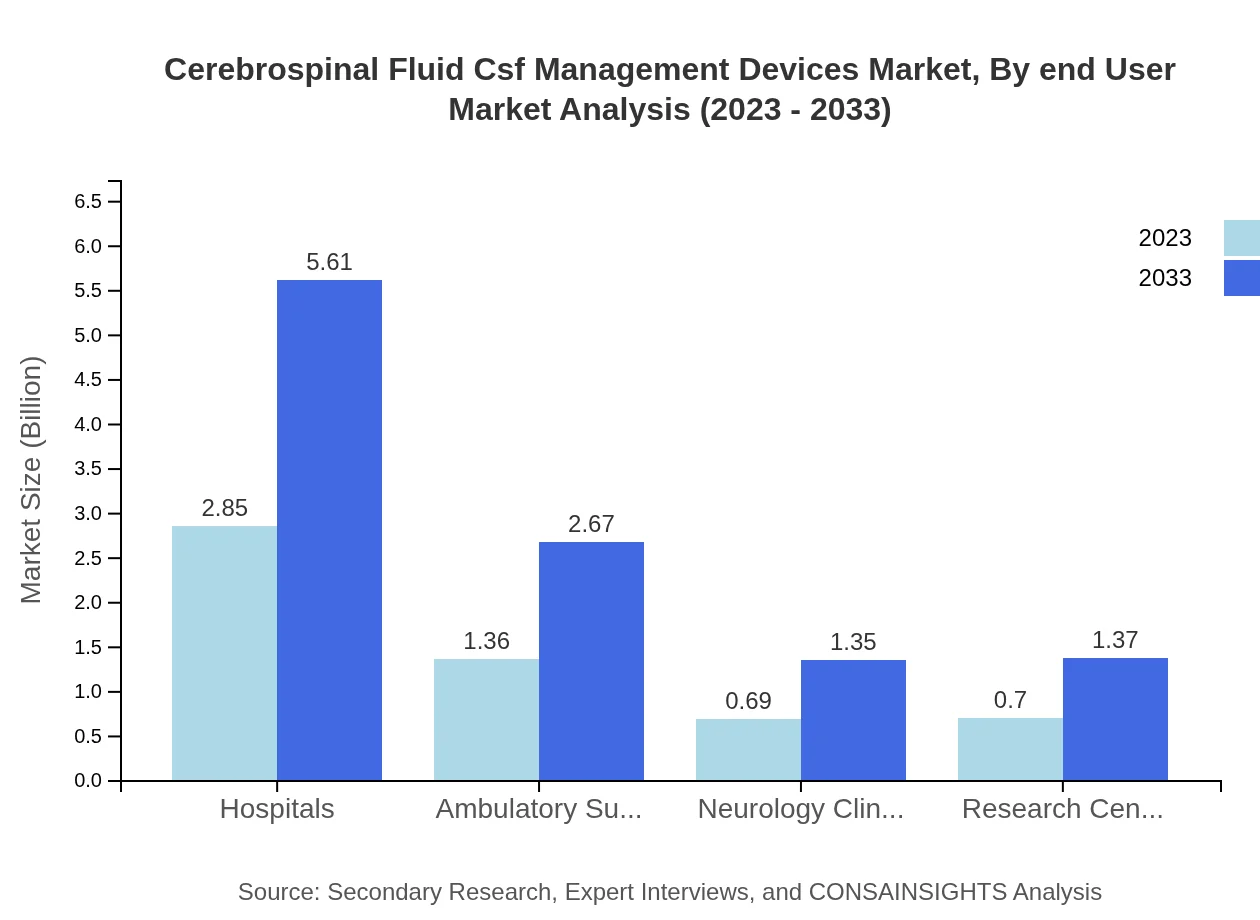
Cerebrospinal Fluid Csf Management Devices Market Analysis By End User
End-user segmentation reveals that hospitals are the largest market segment, expected to maintain their dominance with a market size growing from USD 2.85 billion to USD 5.61 billion. Ambulatory Surgical Centers and neurology clinics are also critical segments with respective market sizes of USD 1.36 billion and USD 0.69 billion in 2023, projected to grow steadily.
Cerebrospinal Fluid Csf Management Devices Market Analysis By Region Type
Regional analysis indicates North America as the dominant market, reflecting a strong share of 47.09% in 2023. Europe follows with a 22.48% share. Asia-Pacific and Latin America possess lower shares at 10.24% and 10.16%, respectively, but are expected to show strong growth as local healthcare sectors improve.
Cerebrospinal Fluid Csf Management Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cerebrospinal Fluid Csf Management Devices Industry
Medtronic :
A global leader in medical device technology, Medtronic specializes in a wide range of neurosurgical products, including advanced shunt systems and stimulation devices.Abbott Laboratories:
Involved in the development of innovative solutions for CSF management, Abbott focuses on creating effective monitoring and management devices for neurological applications.B. Braun Melsungen AG:
This company offers a comprehensive range of medical and pharmaceutical products, including highly regarded CSF management devices that aid in effective treatment protocols.Codman Neuro:
A subsidiary of Integra LifeSciences, Codman Neuro specializes in neurosurgery products, including shunts and reservoirs essential for CSF management.Stryker :
With strong expertise in neurosurgical technologies, Stryker develops and markets a variety of devices aimed at improving CSF management and patient outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of cerebrospinal Fluid Csf Management Devices?
The global market size for cerebrospinal fluid management devices is projected to reach approximately $5.6 billion by 2033, growing at a CAGR of 6.8% from 2023 to 2033.
What are the key market players or companies in this cerebrospinal Fluid Csf Management Devices industry?
Key players in the cerebrospinal fluid management devices market include Medtronic, B. Braun Melsungen AG, Johnson & Johnson, and Smiths Medical, which are prominent in developing innovative solutions.
What are the primary factors driving the growth in the cerebrospinal Fluid Csf Management Devices industry?
Factors driving market growth include the increasing prevalence of neurological disorders, advancements in medical technology, and a rise in awareness regarding cerebrospinal fluid management.
Which region is the fastest Growing in the cerebrospinal Fluid Csf Management Devices market?
The fastest-growing region is Europe, projected to grow from $1.42 billion in 2023 to $2.80 billion by 2033, benefiting from enhanced healthcare infrastructure and rising patient populations.
Does ConsaInsights provide customized market report data for the cerebrospinal Fluid Csf Management Devices industry?
Yes, ConsaInsights offers customized market reports tailored to the cerebrospinal fluid management devices industry, enabling businesses to make informed decisions based on specific requirements.
What deliverables can I expect from this cerebrospinal Fluid Csf Management Devices market research project?
Deliverables include comprehensive market analysis, segmentation data, growth forecasts, competitive landscape assessments, and insights on regional trends and innovations.
What are the market trends of cerebrospinal Fluid Csf Management Devices?
Current trends include increasing adoption of minimally invasive procedures, innovation in device technology, and rising demand for effective management solutions for spinal disorders.