Cervical Artificial Discs Market Report
Published Date: 31 January 2026 | Report Code: cervical-artificial-discs
Cervical Artificial Discs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cervical Artificial Discs market, examining market sizes, trends, and forecasts from 2023 to 2033, alongside regional insights and the competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
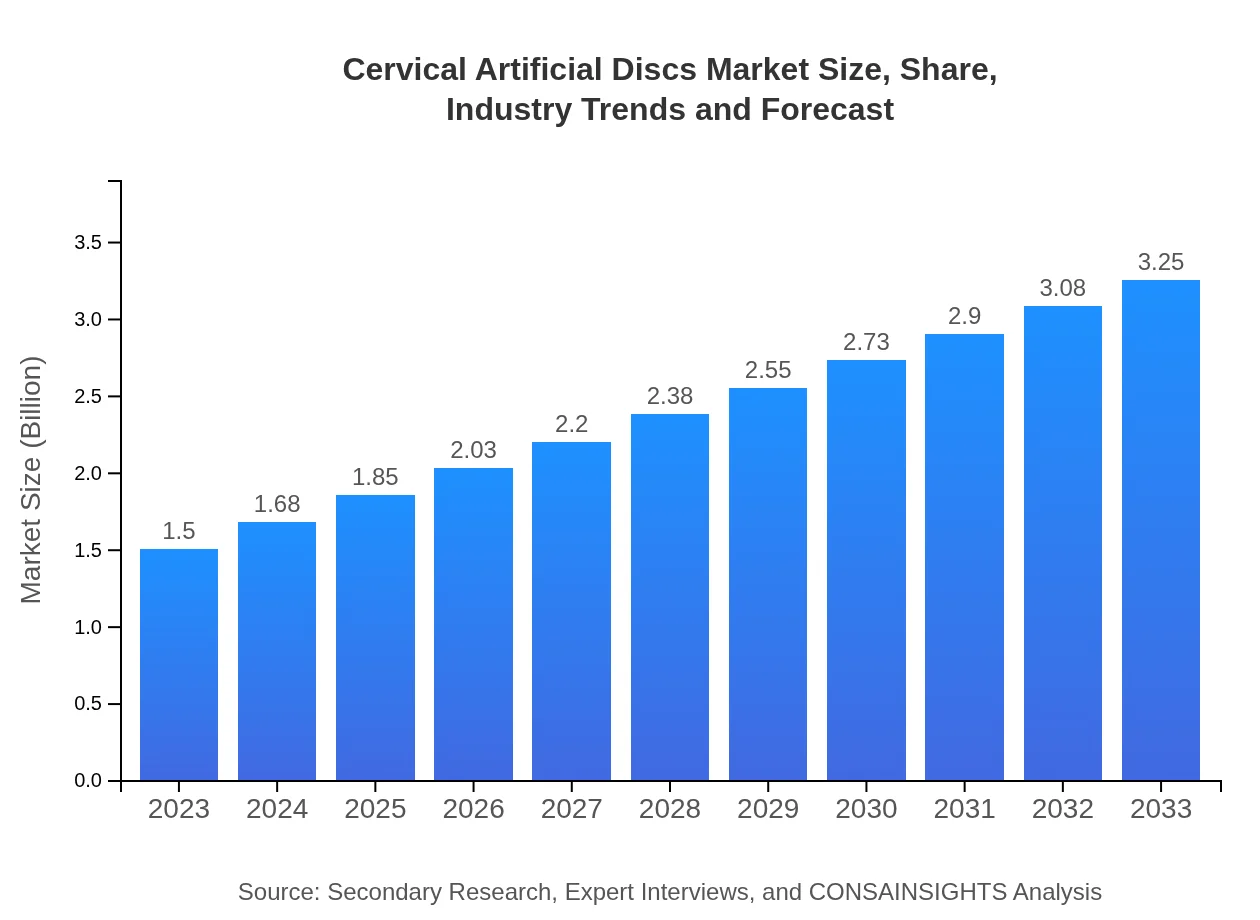
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $3.25 Billion |
| Top Companies | Medtronic , Zimmer Biomet, NuVasive, Globus Medical |
| Last Modified Date | 31 January 2026 |
Cervical Artificial Discs Market Overview
Customize Cervical Artificial Discs Market Report market research report
- ✔ Get in-depth analysis of Cervical Artificial Discs market size, growth, and forecasts.
- ✔ Understand Cervical Artificial Discs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cervical Artificial Discs
What is the Market Size & CAGR of Cervical Artificial Discs market in 2023?
Cervical Artificial Discs Industry Analysis
Cervical Artificial Discs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cervical Artificial Discs Market Analysis Report by Region
Europe Cervical Artificial Discs Market Report:
In Europe, the market size is forecasted to grow from USD 0.42 billion in 2023 to USD 0.92 billion by 2033. This growth is supported by a well-established healthcare system, ongoing research initiatives, and increasing awareness of alternative treatment options.Asia Pacific Cervical Artificial Discs Market Report:
In the Asia Pacific, the market is expected to grow from USD 0.28 billion in 2023 to USD 0.62 billion in 2033. This region is witnessing a surge in healthcare expenditure and improvements in the medical infrastructure, boosting the adoption of cervical artificial discs.North America Cervical Artificial Discs Market Report:
North America holds a significant share, expected to grow from USD 0.57 billion in 2023 to USD 1.23 billion in 2033. Key factors driving this growth include advanced healthcare technology, higher surgical rates, and the presence of numerous market players.South America Cervical Artificial Discs Market Report:
The South American market shows substantial growth potential, with projections increasing from USD 0.12 billion in 2023 to USD 0.26 billion in 2033, driven by the rising prevalence of degenerative diseases and improved access to healthcare services.Middle East & Africa Cervical Artificial Discs Market Report:
The Middle East and Africa market is poised to expand from USD 0.10 billion in 2023 to USD 0.23 billion in 2033, driven by improving healthcare infrastructure and rising investment in medical technologies within the region.Tell us your focus area and get a customized research report.
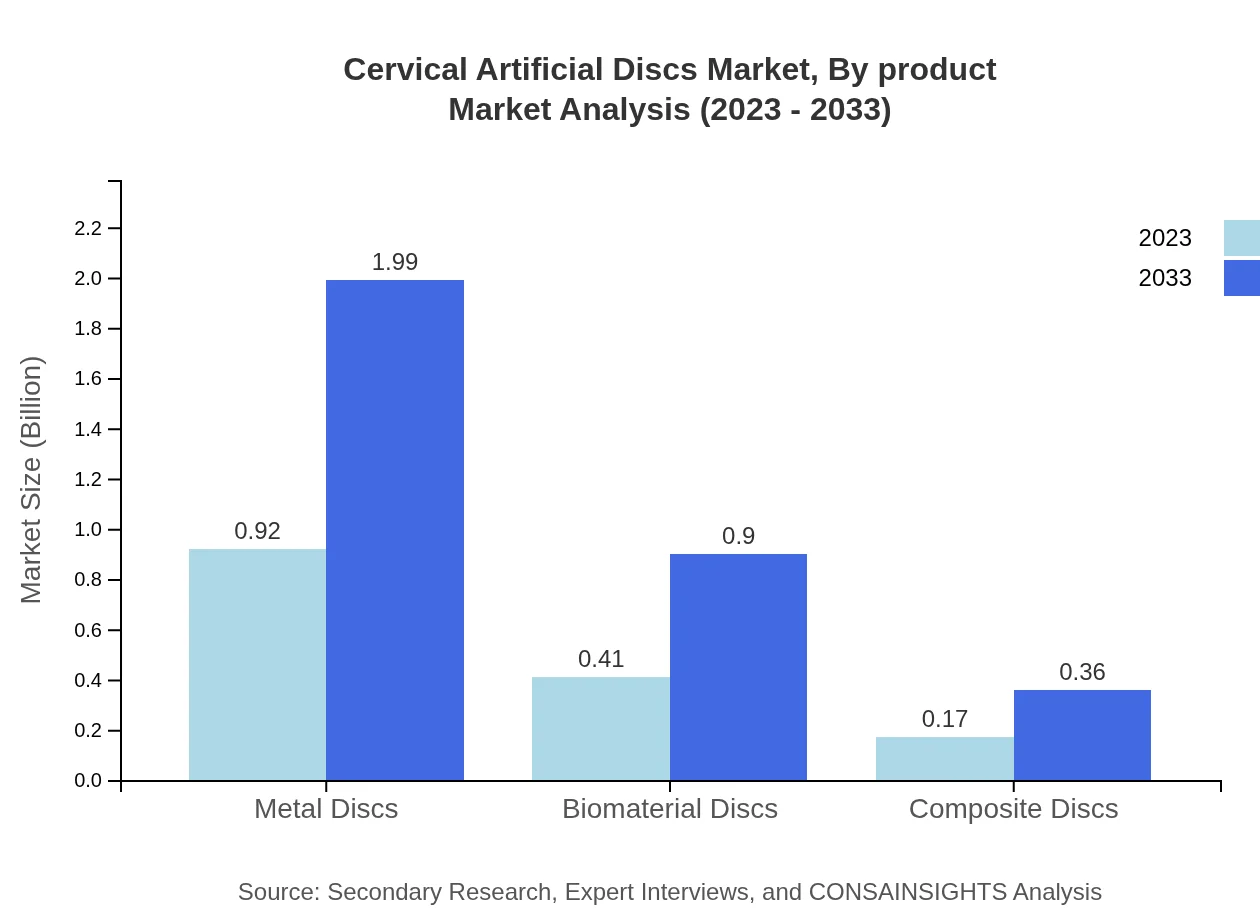
Cervical Artificial Discs Market Analysis By Product
The market is primarily divided into metal discs, biomaterial discs, and composite discs. Metal discs dominate the segment, expected to grow from USD 0.92 billion in 2023 to USD 1.99 billion in 2033. Biomaterial discs are forecasted to expand from USD 0.41 billion to USD 0.90 billion, while composite discs are anticipated to rise from USD 0.17 billion to USD 0.36 billion.
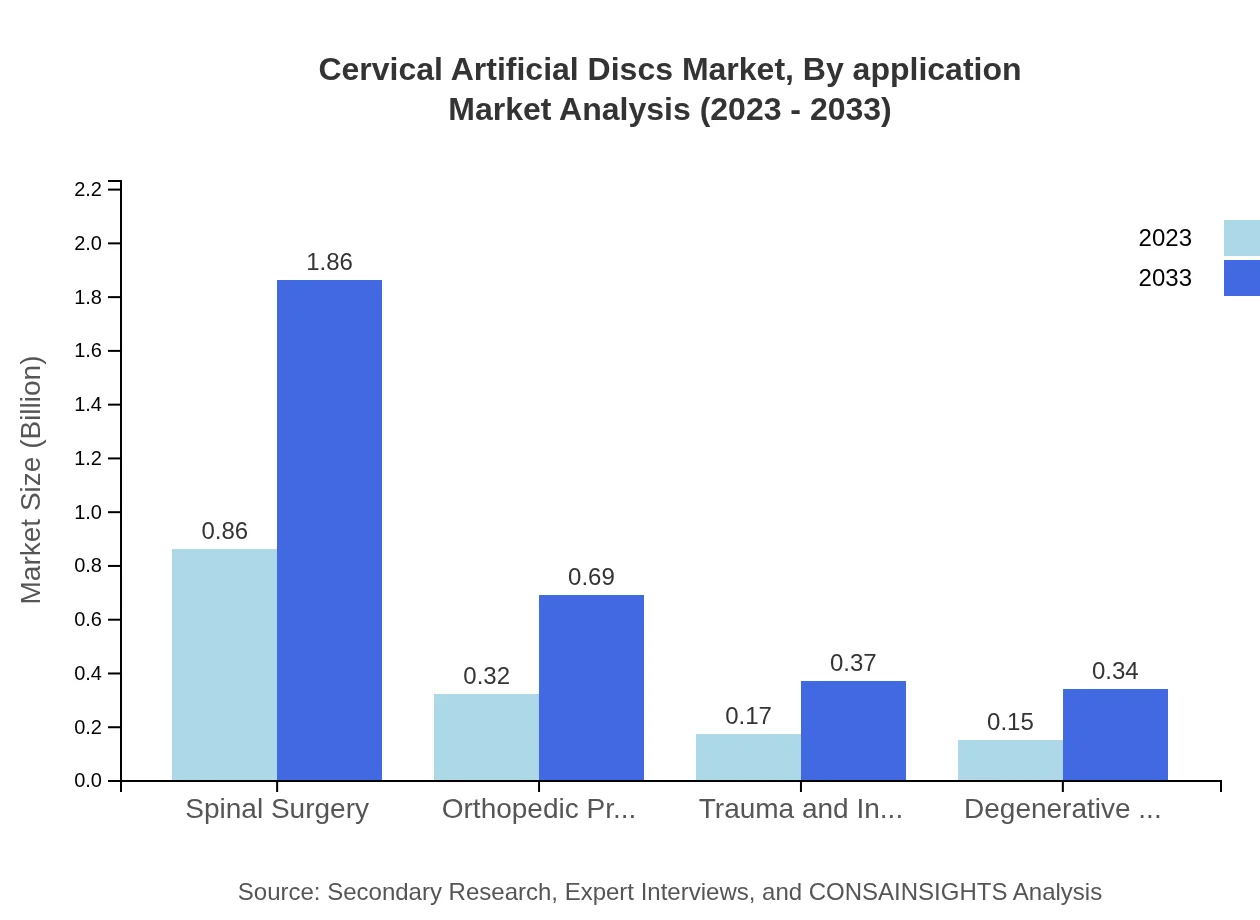
Cervical Artificial Discs Market Analysis By Application
Applications of cervical artificial discs are categorized into spinal surgery, orthopedic procedures, trauma and injury treatments, and degenerative disease treatments. Spinal surgery accounts for the largest market share, expected to reach USD 1.86 billion by 2033 from USD 0.86 billion in 2023. Other applications like orthopedic procedures and trauma treatments will also see significant growth.
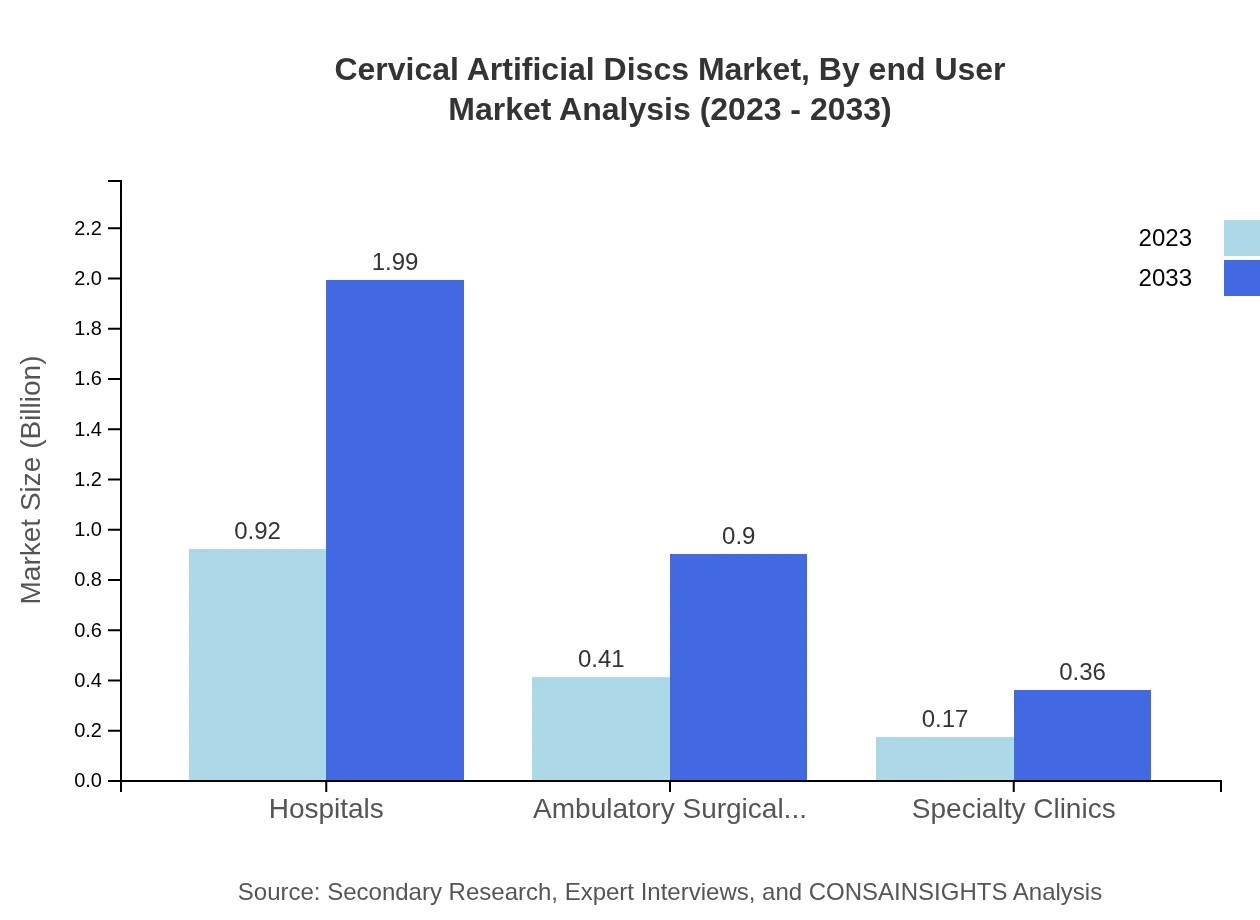
Cervical Artificial Discs Market Analysis By End User
The major end-users include hospitals, ambulatory surgical centers, and specialty clinics. Hospitals represent the largest share, growing from USD 0.92 billion to USD 1.99 billion in 2033. Ambulatory surgical centers are also expected to expand significantly, reflecting shifts in healthcare delivery towards outpatient settings.
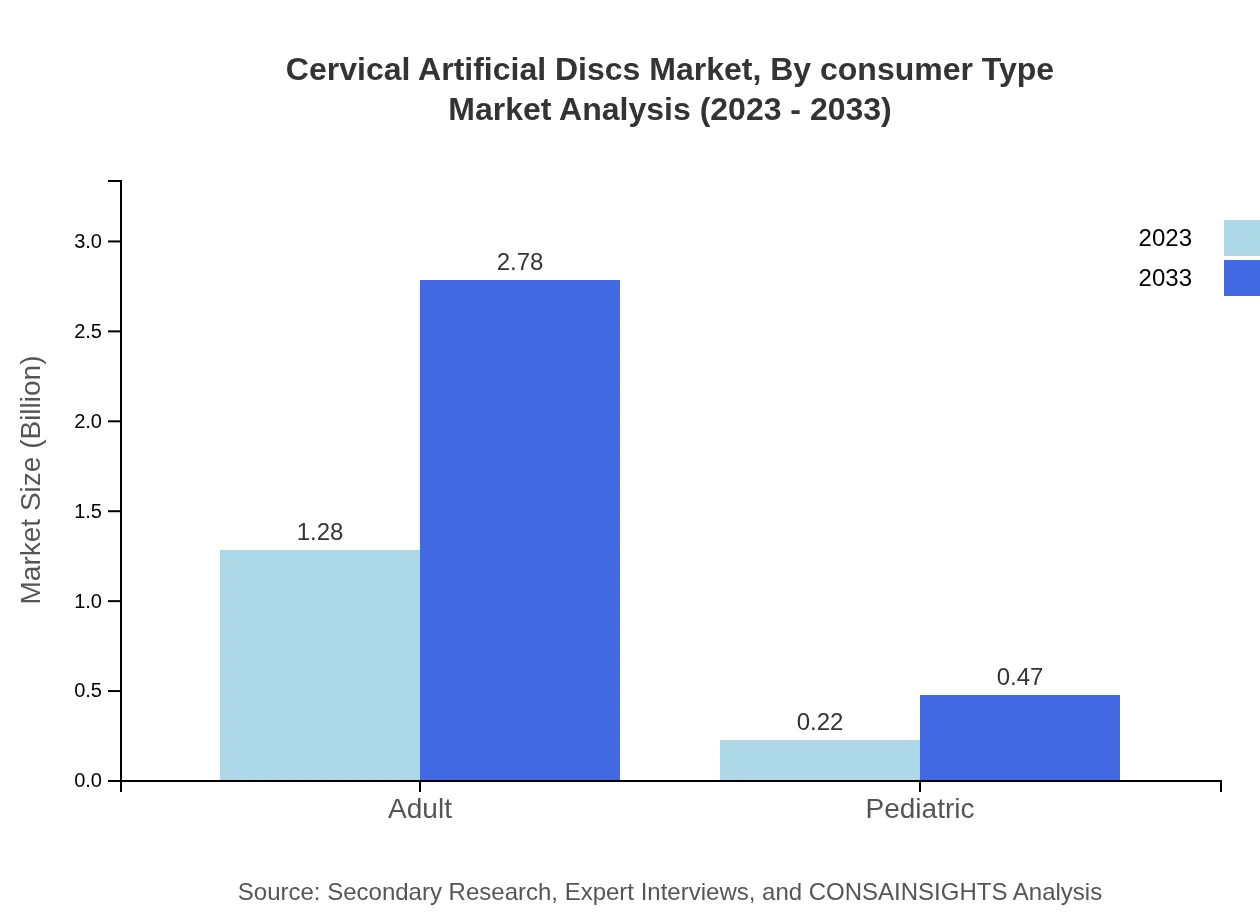
Cervical Artificial Discs Market Analysis By Consumer Type
Consumer types are segmented into adult and pediatric markets. The adult segment constitutes the bulk of the market, projected to grow from USD 1.28 billion in 2023 to USD 2.78 billion by 2033. The pediatric segment, while smaller, is also set for growth from USD 0.22 billion to USD 0.47 billion.
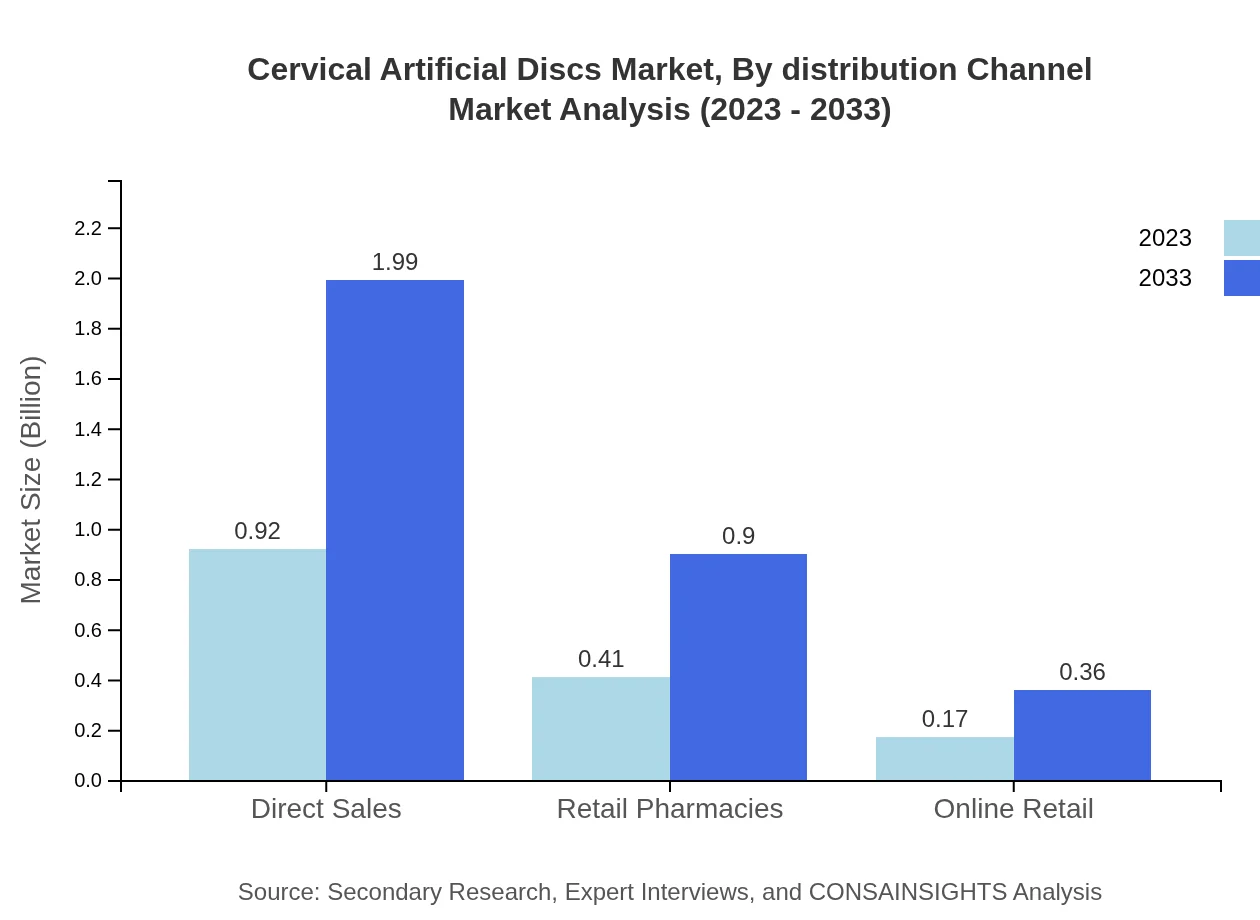
Cervical Artificial Discs Market Analysis By Distribution Channel
Distribution channels include direct sales, retail pharmacies, and online retail. Direct sales dominate this segment, expected to rise from USD 0.92 billion to USD 1.99 billion over the forecast period. Retail pharmacies and online sales channels also contribute growing shares to the overall market.
Cervical Artificial Discs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cervical Artificial Discs Industry
Medtronic :
A leading global medical technology company specializing in devices that treat spinal disorders, including innovative cervical artificial discs.Zimmer Biomet:
A prominent player in the orthopedic and spinal markets, offering a range of cervical artificial discs designed for enhanced patient compatibility.NuVasive:
Known for its focus on minimally invasive surgical solutions, NuVasive specializes in advanced cervical disc systems that promote safety and efficacy.Globus Medical:
A key innovator in spinal surgery technologies, Globus Medical provides cervical artificial discs that utilize state-of-the-art design and materials.We're grateful to work with incredible clients.









FAQs
What is the market size of cervical artificial discs?
The cervical artificial discs market was valued at approximately $1.5 billion in 2023 and is projected to grow at a CAGR of 7.8%, indicating significant growth potential by 2033.
What are the key market players or companies in the cervical artificial discs industry?
The cervical artificial discs market includes major players such as Medtronic, NuVasive, Johnson & Johnson, and Globus Medical, who are instrumental in shaping market trends and innovations.
What are the primary factors driving the growth in the cervical artificial discs industry?
Key drivers include the rising prevalence of cervical spine disorders, advancements in surgical technologies, and an increasing aging population requiring effective spinal interventions.
Which region is the fastest Growing in the cervical artificial discs market?
North America is the fastest-growing region, with the market expected to expand from $0.57 billion in 2023 to $1.23 billion by 2033, attributed to increased healthcare expenditure and technological advancements.
Does ConsaInsights provide customized market report data for the cervical artificial discs industry?
Yes, ConsaInsights offers tailored market report data focusing on specific needs within the cervical artificial discs industry, enabling detailed insights and informed decision-making.
What deliverables can I expect from this cervical artificial discs market research project?
Deliverables include comprehensive market analysis reports, regional insights, competitor analysis, trend forecasts, and segment-specific data tailored to your requirements.
What are the market trends of cervical artificial discs?
Current trends indicate a shift towards minimally invasive surgical techniques, increasing use of biomaterial discs, and a growing focus on patient-specific treatment approaches in the cervical artificial discs market.