Cervical Cancer Diagnostics And Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: cervical-cancer-diagnostics-and-therapeutics
Cervical Cancer Diagnostics And Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Cervical Cancer Diagnostics and Therapeutics market, detailing current trends, market size, segmentation, and forecasts for the years 2023 to 2033. Insights include regional performance, product types, technological advancements, and the competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
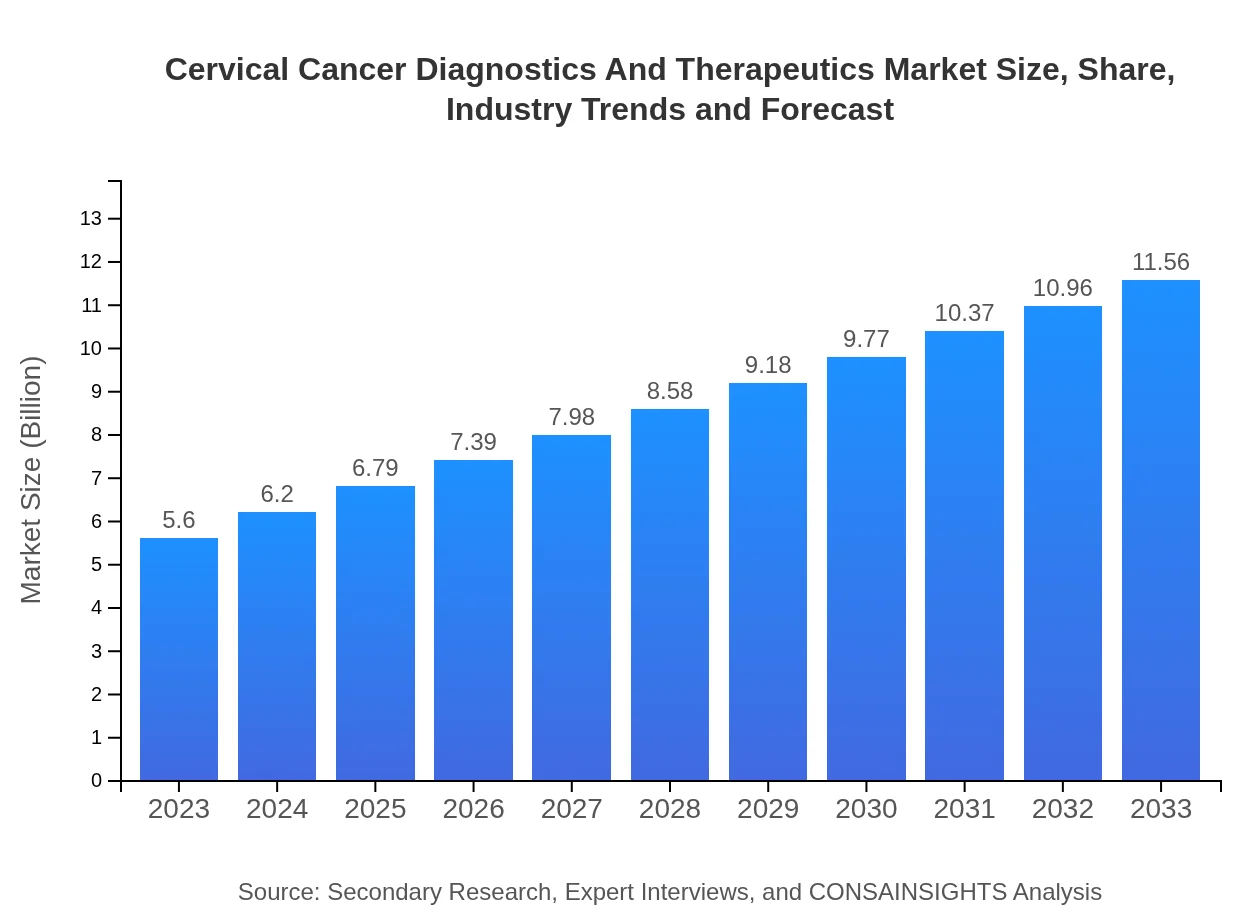
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.3% |
| 2033 Market Size | $11.56 Billion |
| Top Companies | Roche, Bristol-Myers Squibb, Merck & Co., Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Cervical Cancer Diagnostics And Therapeutics Market Overview
Customize Cervical Cancer Diagnostics And Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Cervical Cancer Diagnostics And Therapeutics market size, growth, and forecasts.
- ✔ Understand Cervical Cancer Diagnostics And Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cervical Cancer Diagnostics And Therapeutics
What is the Market Size & CAGR of Cervical Cancer Diagnostics And Therapeutics market in 2023?
Cervical Cancer Diagnostics And Therapeutics Industry Analysis
Cervical Cancer Diagnostics And Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cervical Cancer Diagnostics And Therapeutics Market Analysis Report by Region
Europe Cervical Cancer Diagnostics And Therapeutics Market Report:
With a market size of $1.51 billion in 2023, Europe is expected to grow to $3.12 billion by 2033. Significant investments in healthcare, along with government initiatives to reduce cervical cancer rates, drive the market.Asia Pacific Cervical Cancer Diagnostics And Therapeutics Market Report:
In 2023, the Asia-Pacific market is valued at $1.18 billion, projected to grow to $2.43 billion by 2033. Heightened awareness about cervical cancer combined with improving healthcare infrastructure boosts demand for diagnostics and therapeutics in this region.North America Cervical Cancer Diagnostics And Therapeutics Market Report:
North America held the lead in 2023 with a market size of $1.92 billion, and is projected to reach $3.96 billion by 2033. Increased funding for cancer research and advanced healthcare services underpin this growth.South America Cervical Cancer Diagnostics And Therapeutics Market Report:
The South America market, worth $0.44 billion in 2023, is expected to double to $0.91 billion by 2033. Growing detection rates and advancements in affordable healthcare solutions are pivotal for future growth.Middle East & Africa Cervical Cancer Diagnostics And Therapeutics Market Report:
The market in the Middle East and Africa, valued at $0.55 billion in 2023, is anticipated to grow to $1.14 billion by 2033. Increasing awareness of cervical cancer and regional health policies are driving factors.Tell us your focus area and get a customized research report.
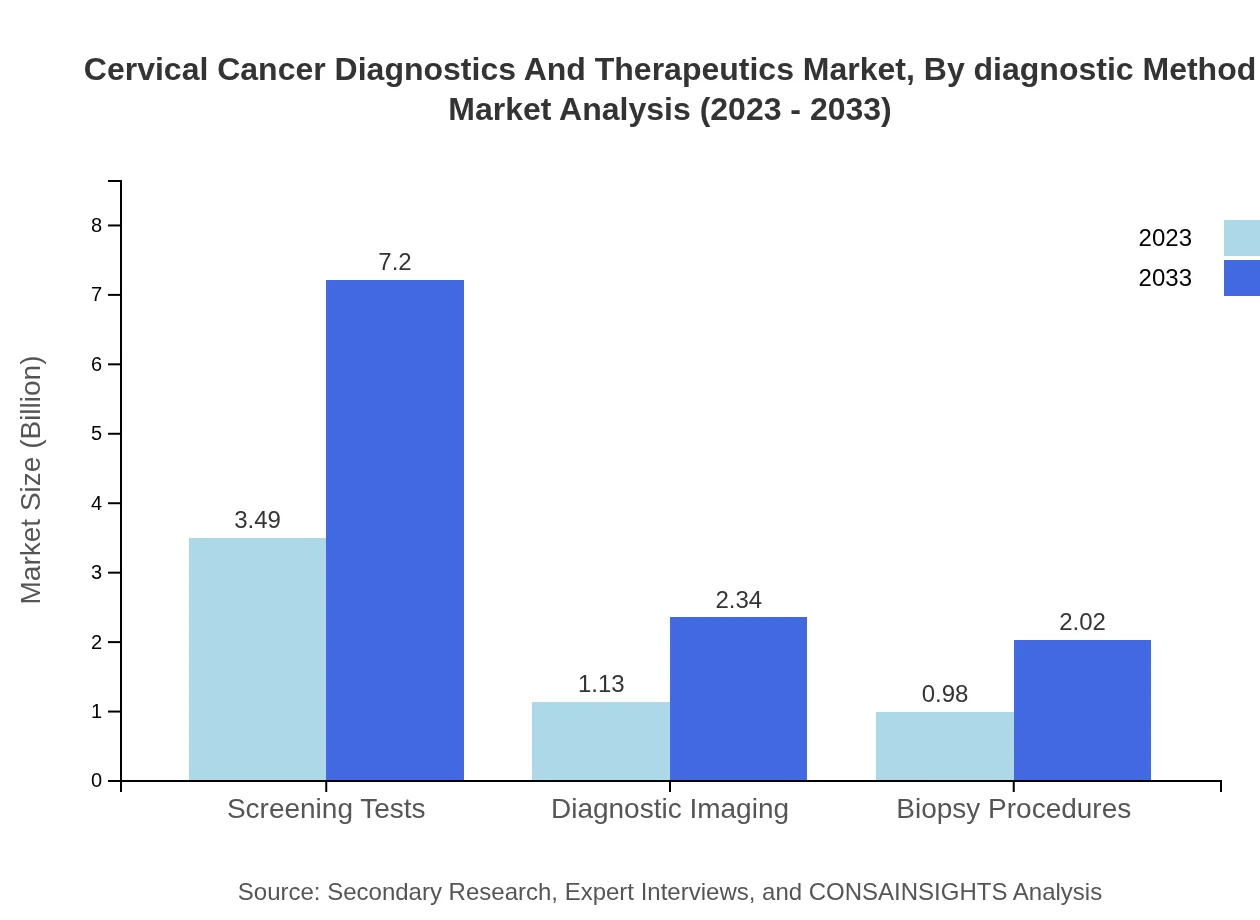
Cervical Cancer Diagnostics And Therapeutics Market Analysis By Diagnostic Method
In 2023, the diagnostic segment is primarily dominated by screening tests, which account for $3.49 billion, projected to grow to $7.20 billion by 2033. Imaging and biopsy procedures also contribute significantly with sales expected to rise from $1.13 billion to $2.34 billion and from $0.98 billion to $2.02 billion, respectively, providing diverse approaches for cervical cancer diagnosis.
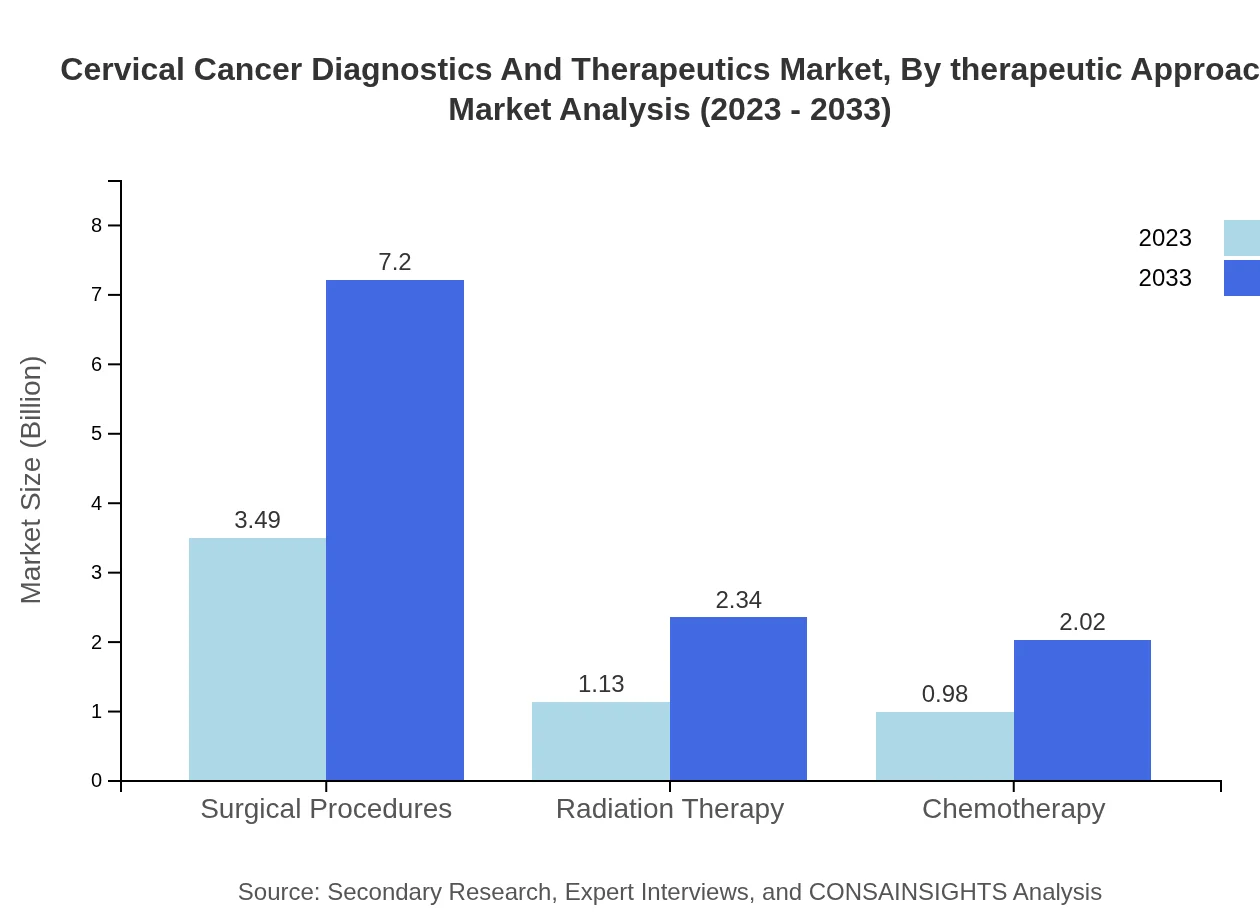
Cervical Cancer Diagnostics And Therapeutics Market Analysis By Therapeutic Approach
Targeted therapy is set to retain dominance in the therapeutics market, attaining $3.49 billion in 2023 and expected to reach $7.20 billion by 2033. Immunotherapy and hormonal therapy show considerable growth from $1.13 billion to $2.34 billion and $0.98 billion to $2.02 billion, indicating substantial advancements in treatment options.
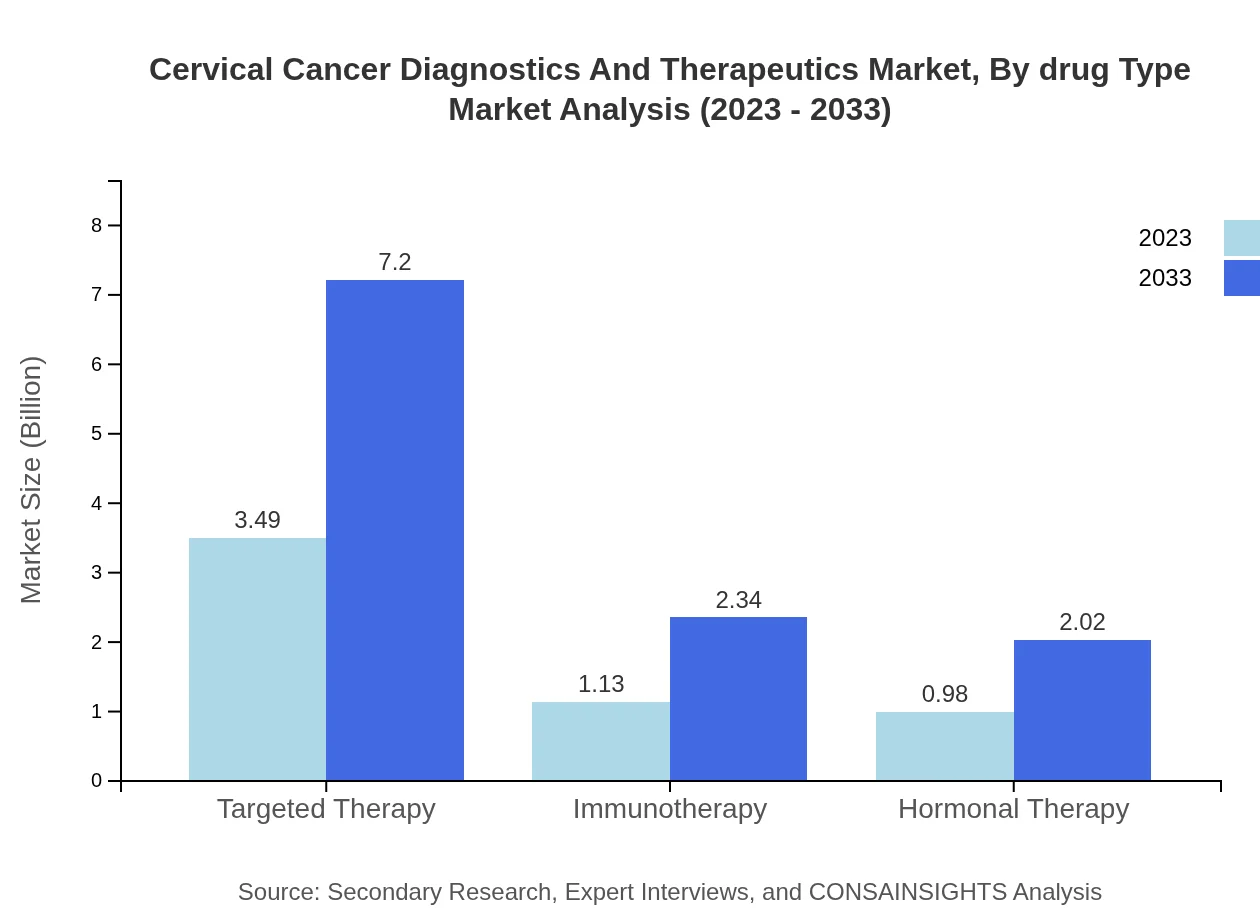
Cervical Cancer Diagnostics And Therapeutics Market Analysis By Drug Type
The drug type segment shows that chemotherapy remains a pertinent option in cervical cancer treatment, starting at a market value of $0.98 billion in 2023, and projected to grow to $2.02 billion by 2033. Recent drug developments are significantly enhancing therapeutic efficacy.
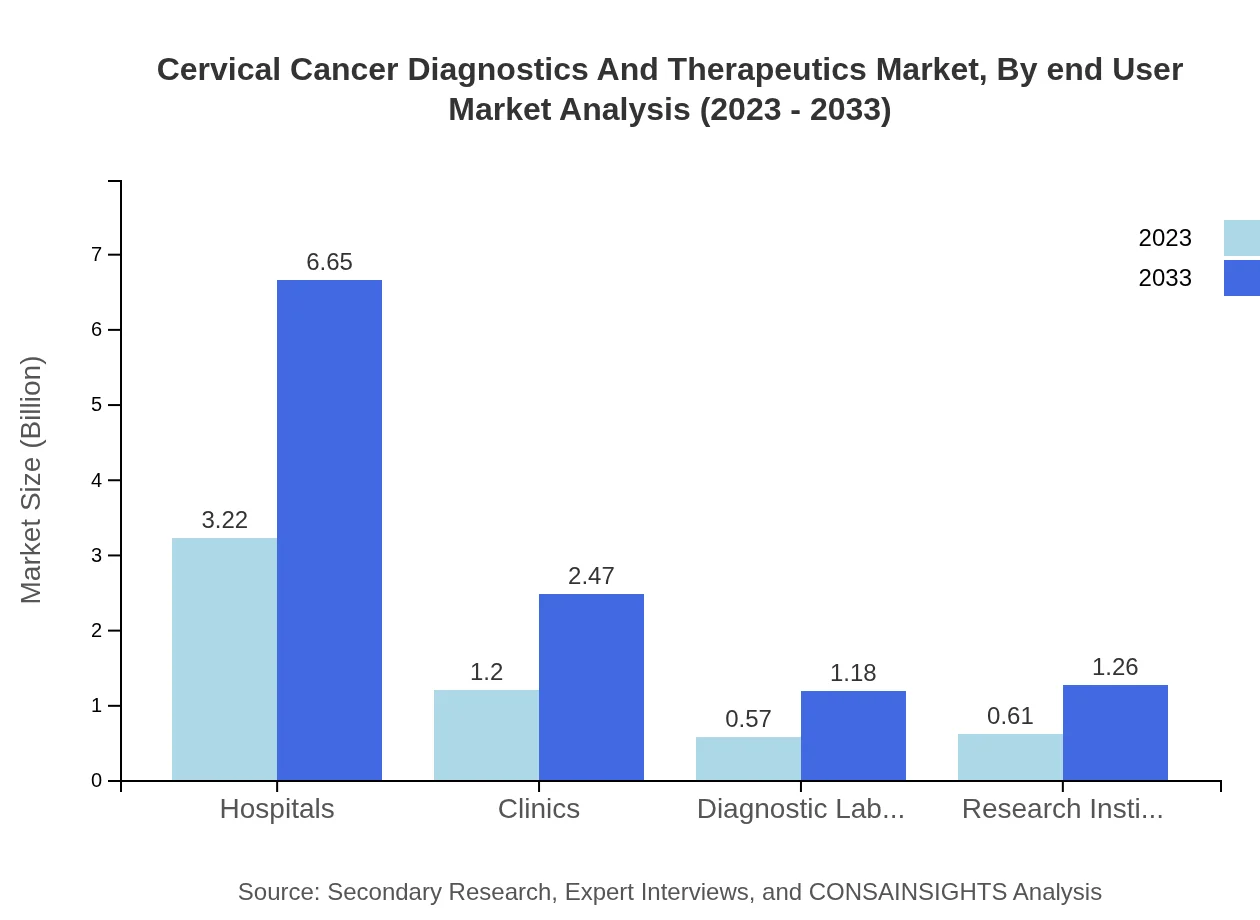
Cervical Cancer Diagnostics And Therapeutics Market Analysis By End User
The diagnostic laboratories and hospitals dominate the end-user market, forecasting to maintain a share of 57.5% by 2033. Hospitals are expected to cater to over 50% of the market through enhanced diagnostic and treatment facilities.
Cervical Cancer Diagnostics And Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cervical Cancer Diagnostics And Therapeutics Industry
Roche:
A leader in cancer diagnostics, Roche offers innovative HPV tests and treatment solutions that significantly improve diagnostic accuracy and patient outcomes.Bristol-Myers Squibb:
Focusing on immuno-oncology, Bristol-Myers Squibb provides advanced therapies that enhance the immune system's ability to combat cervical cancer.Merck & Co.:
Known for their HPV vaccine, Merck also develops therapeutic treatments for cervical cancer and invests heavily in research for better outcomes.Siemens Healthineers:
Siemens Healthineers offers comprehensive imaging and diagnostics solutions that are pivotal in the early detection of cervical cancer.We're grateful to work with incredible clients.









FAQs
What is the market size of cervical Cancer Diagnostics And Therapeutics?
The cervical cancer diagnostics and therapeutics market is projected to reach USD 5.6 billion by 2033, growing at a CAGR of 7.3% from 2023. This growth is primarily driven by advances in diagnostic technologies and therapeutic approaches.
What are the key market players or companies in this cervical Cancer Diagnostics And Therapeutics industry?
Key players in the cervical cancer diagnostics and therapeutics market include Roche, Abbott Laboratories, Merck & Co., Hologic, and Siemens Healthineers, among others. These companies are focusing on innovation and partnerships to strengthen their market position.
What are the primary factors driving the growth in the cervical Cancer Diagnostics And Therapeutics industry?
Factors driving growth include rising cervical cancer incidence, advancements in testing and treatment technologies, increased public awareness about screening, and supportive government initiatives for vaccination and preventive measures.
Which region is the fastest Growing in the cervical Cancer Diagnostics And Therapeutics?
The Asia Pacific region is poised to be the fastest-growing market, expanding from USD 1.18 billion in 2023 to USD 2.43 billion by 2033. This growth is spurred by improving healthcare infrastructure and rising awareness about cervical cancer screening.
Does ConsaInsights provide customized market report data for the cervical Cancer Diagnostics And Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs within the cervical cancer diagnostics and therapeutics industry, ensuring relevant insights and strategies for informed decision-making.
What deliverables can I expect from this cervical Cancer Diagnostics And Therapeutics market research project?
Deliverables include detailed market analysis reports, competitive landscape assessments, trend evaluations, growth forecasts, and strategic recommendations tailored to the cervical cancer diagnostics and therapeutics sector.
What are the market trends of cervical Cancer Diagnostics And Therapeutics?
Key trends include increasing adoption of minimally invasive diagnostic techniques, growth in personalized therapies, expanding screening programs, and a boost in telemedicine services for patient engagement and follow-ups.