Cervical Forceps Market Report
Published Date: 31 January 2026 | Report Code: cervical-forceps
Cervical Forceps Market Size, Share, Industry Trends and Forecast to 2033
This market report explores the global Cervical Forceps market, providing insights into its size, growth, and key trends from 2023 to 2033. Detailed analyses of regional markets, segmentation, technology advancements, and leading companies will aid stakeholders in making informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
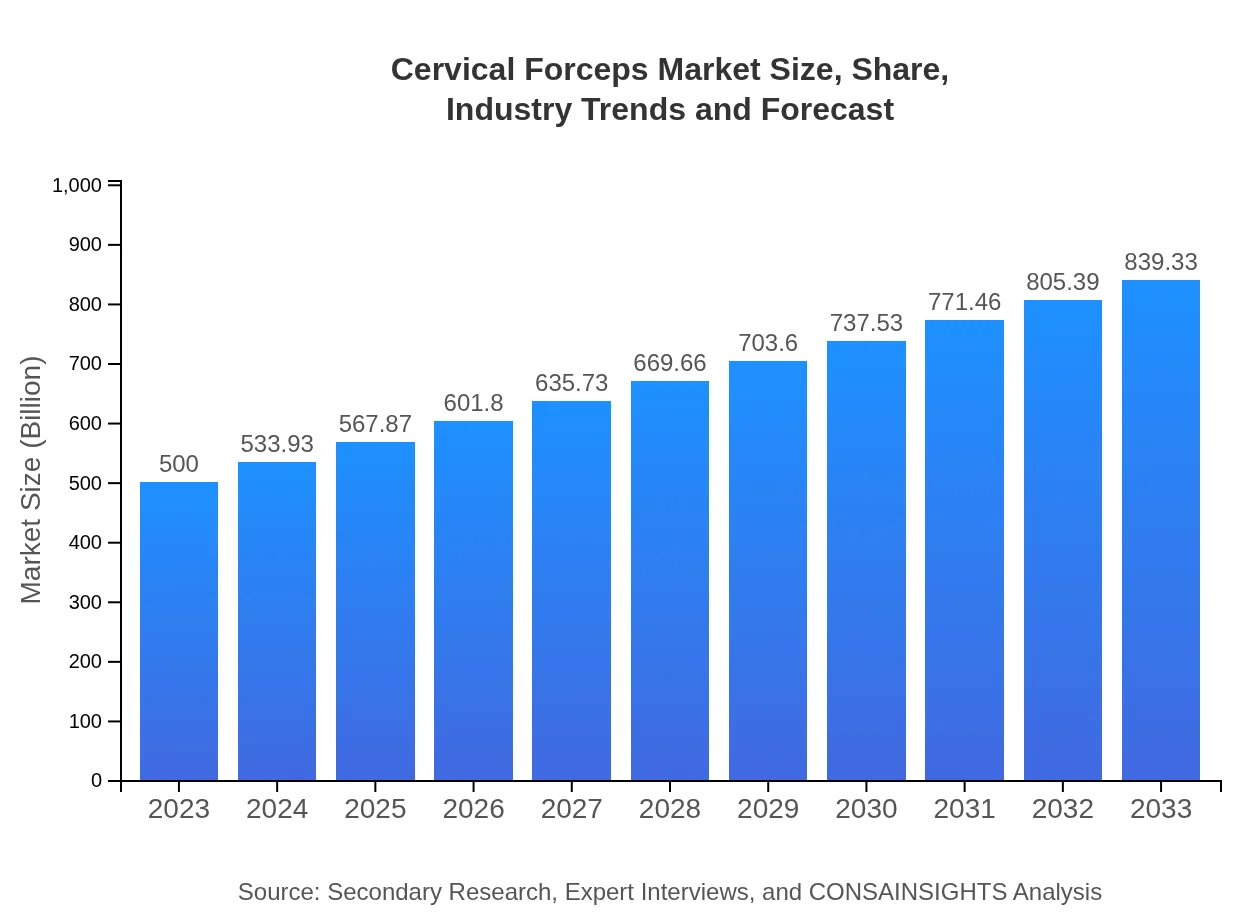
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.2% |
| 2033 Market Size | $839.33 Million |
| Top Companies | Medtronic , B. Braun Melsungen AG, Johnson & Johnson, Stryker Corporation, Cook Medical |
| Last Modified Date | 31 January 2026 |
Cervical Forceps Market Overview
Customize Cervical Forceps Market Report market research report
- ✔ Get in-depth analysis of Cervical Forceps market size, growth, and forecasts.
- ✔ Understand Cervical Forceps's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cervical Forceps
What is the Market Size & CAGR of Cervical Forceps market in 2023?
Cervical Forceps Industry Analysis
Cervical Forceps Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cervical Forceps Market Analysis Report by Region
Europe Cervical Forceps Market Report:
The European Cervical Forceps market is expected to grow from $145.90 million in 2023 to $244.92 million by 2033. The strong regulatory framework regarding women's health and increasing healthcare accessibility contribute to this growth. Major countries like Germany and France are leading the market owing to their robust healthcare systems and emphasis on innovation.Asia Pacific Cervical Forceps Market Report:
The Asia Pacific region is witnessing rapid growth in the Cervical Forceps market, expected to reach $162.16 million by 2033 from $96.60 million in 2023. This growth is influenced by increasing healthcare investments, rising awareness of women’s health, and a surge in childbirth rates. Emerging economies such as India and China are key contributors to this expansion due to their large population and improving healthcare infrastructure.North America Cervical Forceps Market Report:
North America currently holds the largest share of the Cervical Forceps market, with a projected value of $306.27 million by 2033, up from $182.45 million in 2023. This region benefits from advanced healthcare facilities, a high rate of surgical procedures, and significant investments in medical technologies. The aging population in the U.S. also necessitates more gynecological procedures, fueling market growth.South America Cervical Forceps Market Report:
In South America, the Cervical Forceps market is projected to grow from $42.30 million in 2023 to $71.01 million by 2033. Factors including a growing number of healthcare facilities and increasing healthcare budgets contribute to this growth. The expanding middle class in countries like Brazil and Argentina is also enhancing demand for advanced healthcare solutions.Middle East & Africa Cervical Forceps Market Report:
In the Middle East and Africa, the Cervical Forceps market's growth trajectory involves rising hospital establishments and improved healthcare facilities, expected to grow from $32.75 million in 2023 to $54.98 million by 2033. Factors such as improving gynecological services in GCC countries are driving this growth, alongside governmental efforts to enhance maternal healthcare.Tell us your focus area and get a customized research report.
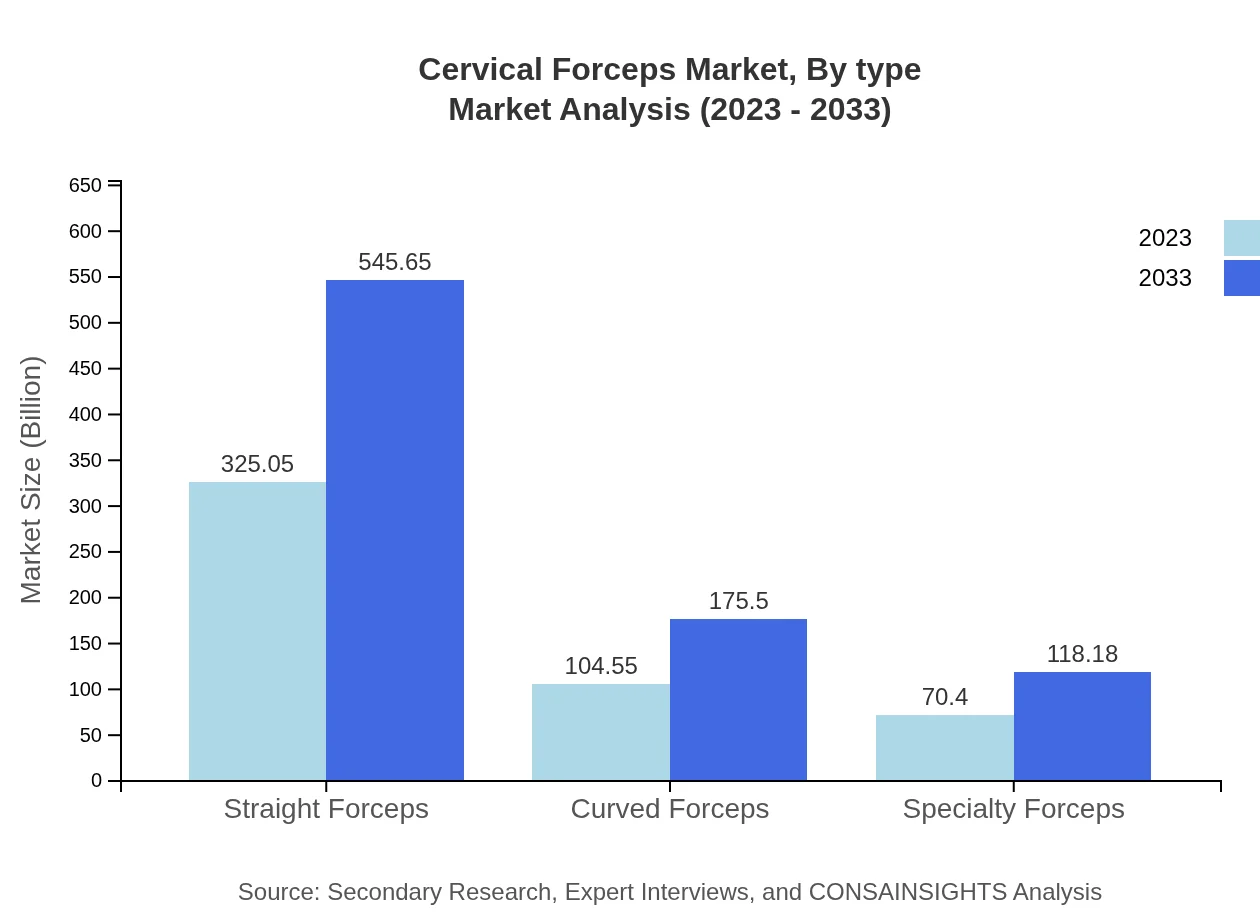
Cervical Forceps Market Analysis By Type
The market is divided into several types: Straight Forceps, Curved Forceps, and Specialty Forceps. Straight Forceps dominate the market with a size of $325.05 million in 2023 and expected to grow to $545.65 million by 2033. Curved Forceps follow with $104.55 million in 2023, projected to reach $175.50 million. Specialty Forceps, while having a smaller market share, are crucial for specialized surgical procedures, expected to grow from $70.40 million to $118.18 million.
Cervical Forceps Market Analysis By Material
Global Cervical Forceps Market, By Material Market Analysis (2023 - 2033)
Material segmentation covers Stainless Steel, Titanium, and Plastic. Stainless Steel holds a significant portion at $325.05 million, reflecting its established use in surgical instruments, with growth anticipated to reach $545.65 million by 2033. Titanium, known for its lightweight properties, is valued at $104.55 million and expected to grow to $175.50 million, while plastic instruments, at $70.40 million, focus on cost-effectiveness and are projected to reach $118.18 million.
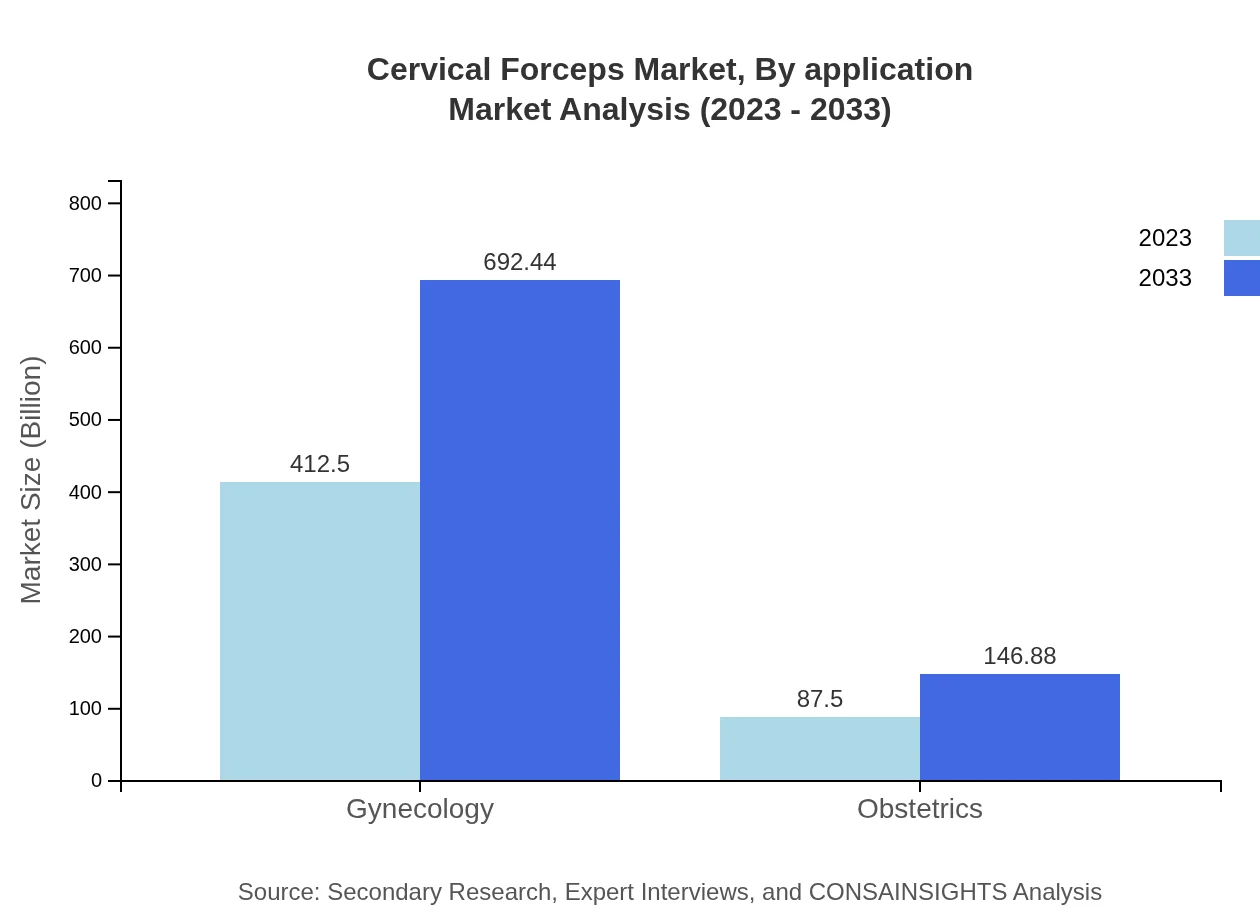
Cervical Forceps Market Analysis By Application
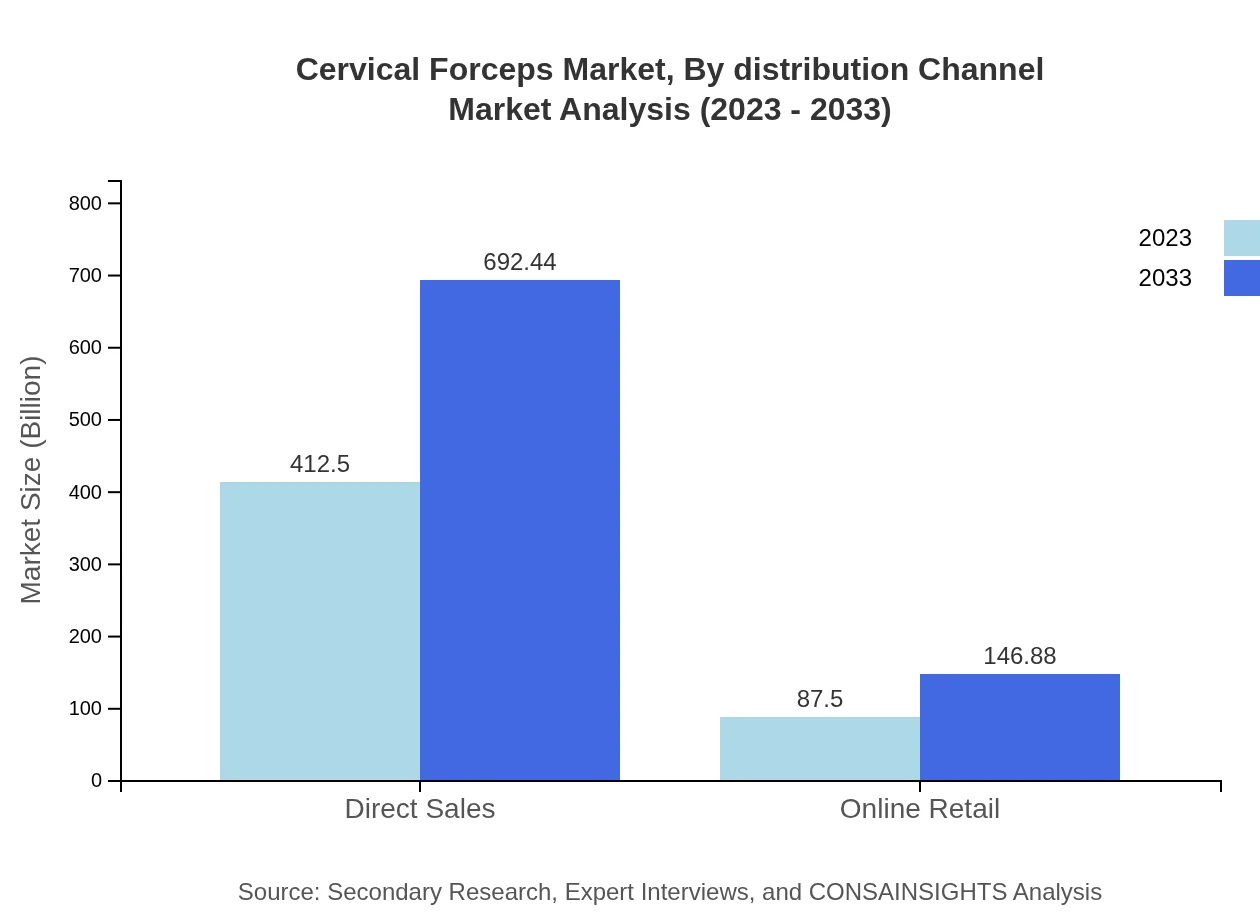
In terms of applications, the market is primarily aligned with Gynecology and Obstetrics. Gynecology dominates with a market size of $412.50 million in 2023 and a forecast of $692.44 million by 2033, indicating strong demand for feminine health care. Obstetrics accounts for $87.50 million currently, projected to improve to $146.88 million, driven by healthcare initiatives focusing on maternal health.
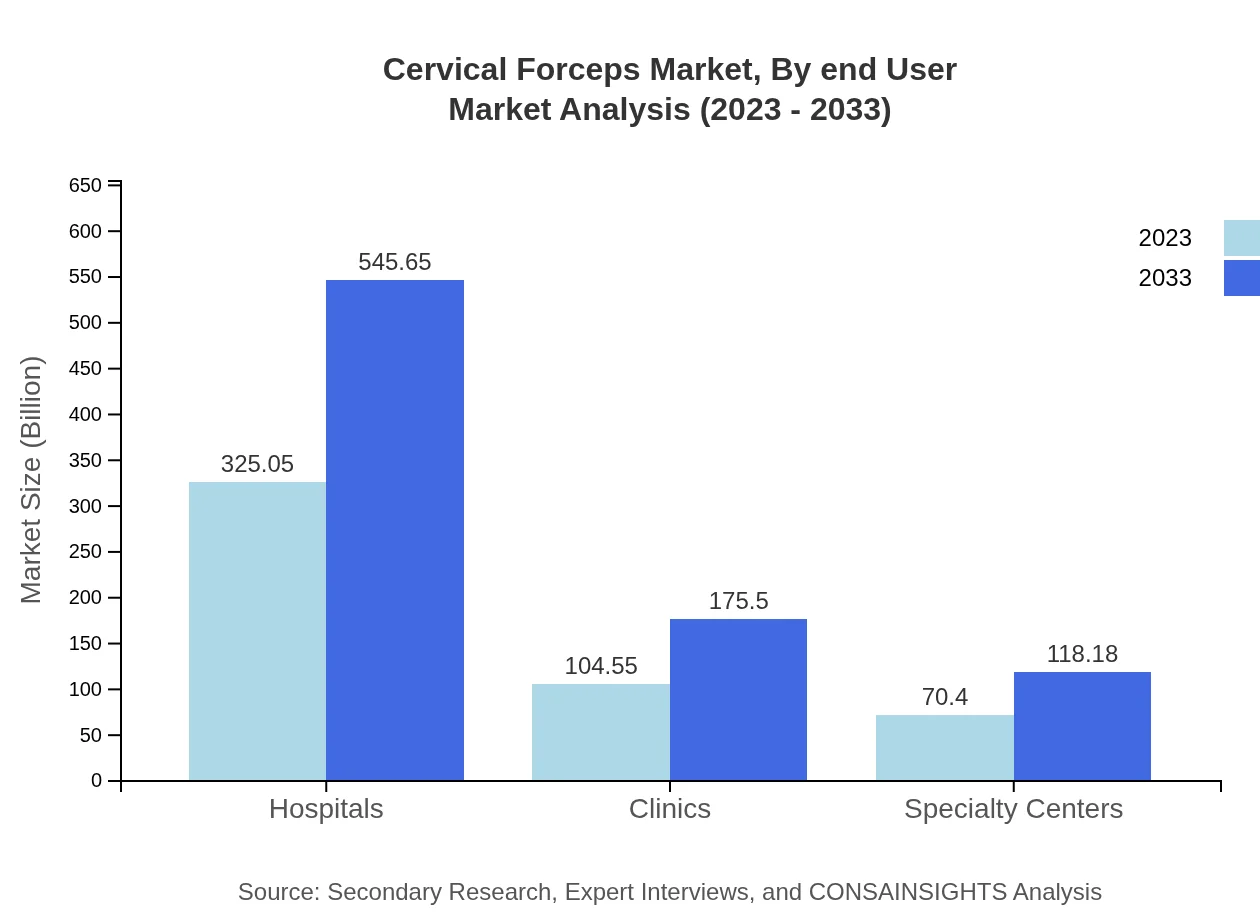
Cervical Forceps Market Analysis By End User
Key end-users are Hospitals, Clinics, and Specialty Centers. Hospitals lead the market with a size of $325.05 million in 2023, expected to reach $545.65 million by 2033. Clinics are seeing growth as well, moving from $104.55 million to $175.50 million. Specialty Centers, crucial for targeted treatments, are also likely to see progress from $70.40 million to $118.18 million.
Cervical Forceps Market Analysis By Distribution Channel
Distribution channels include Direct Sales and Online Retail. Direct Sales dominate with $412.50 million in 2023 and expected growth to $692.44 million by 2033, reinforced by established supplier relationships. Online Retail, while smaller, is emerging with $87.50 million in 2023, projected to rise to $146.88 million, as patients and healthcare providers increasingly turn to online platforms for purchasing.
Cervical Forceps Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cervical Forceps Industry
Medtronic :
Medtronic is a leading global healthcare technology company focusing on medical devices and equipment, including advanced cervical forceps for gynecology.B. Braun Melsungen AG:
B. Braun is known for its high-quality surgical instruments and has a robust lineup of cervical forceps tailored for various gynecological and obstetric procedures.Johnson & Johnson:
Johnson & Johnson provides a wide range of healthcare products, including surgical instruments like cervical forceps, emphasizing innovation and safety in women's health care.Stryker Corporation:
Stryker offers innovative medical devices and instrument solutions, including high-performance cervical forceps, focusing on improving surgical outcomes.Cook Medical:
Cook Medical specializes in medical products for various medical fields, including advanced cervical forceps designed for specific surgical needs.We're grateful to work with incredible clients.









FAQs
What is the market size of cervical Forceps?
The cervical forceps market is valued at approximately $500 million in 2023 and is projected to grow at a CAGR of 5.2%, aiming for significant expansion by 2033.
What are the key market players or companies in the cervical Forceps industry?
Key players in the cervical forceps market include major medical device manufacturers and healthcare companies specializing in surgical instruments, particularly in gynecology and obstetrics sectors.
What are the primary factors driving the growth in the cervical Forceps industry?
The growth factors for the cervical forceps market include increasing gynecological surgeries, advancements in medical technologies, and rising awareness about women's health, leading to higher demand for efficient surgical instruments.
Which region is the fastest Growing in the cervical Forceps?
The cervical forceps market in North America is expected to grow rapidly, from $182.45 million in 2023 to $306.27 million by 2033, driven by advanced healthcare systems and increased surgical procedures.
Does ConsaInsights provide customized market report data for the cervical Forceps industry?
Yes, ConsaInsights offers customized market report data tailored to client needs in the cervical-forceps industry, enabling detailed insights into market dynamics and regional performance.
What deliverables can I expect from this cervical Forceps market research project?
Deliverables from the cervical forceps market research project include comprehensive market analysis, segmentation data, regional insights, and forecasts, ensuring clients receive actionable intelligence.
What are the market trends of cervical Forceps?
Current trends in the cervical forceps market include increasing adoption of minimally invasive surgical techniques, innovations in forcep designs, and a growing preference for single-use devices among healthcare providers.