Checkpoint Inhibitors Market Report
Published Date: 31 January 2026 | Report Code: checkpoint-inhibitors
Checkpoint Inhibitors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Checkpoint Inhibitors market, covering current trends, size, segmentation, and forecasts for the period of 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
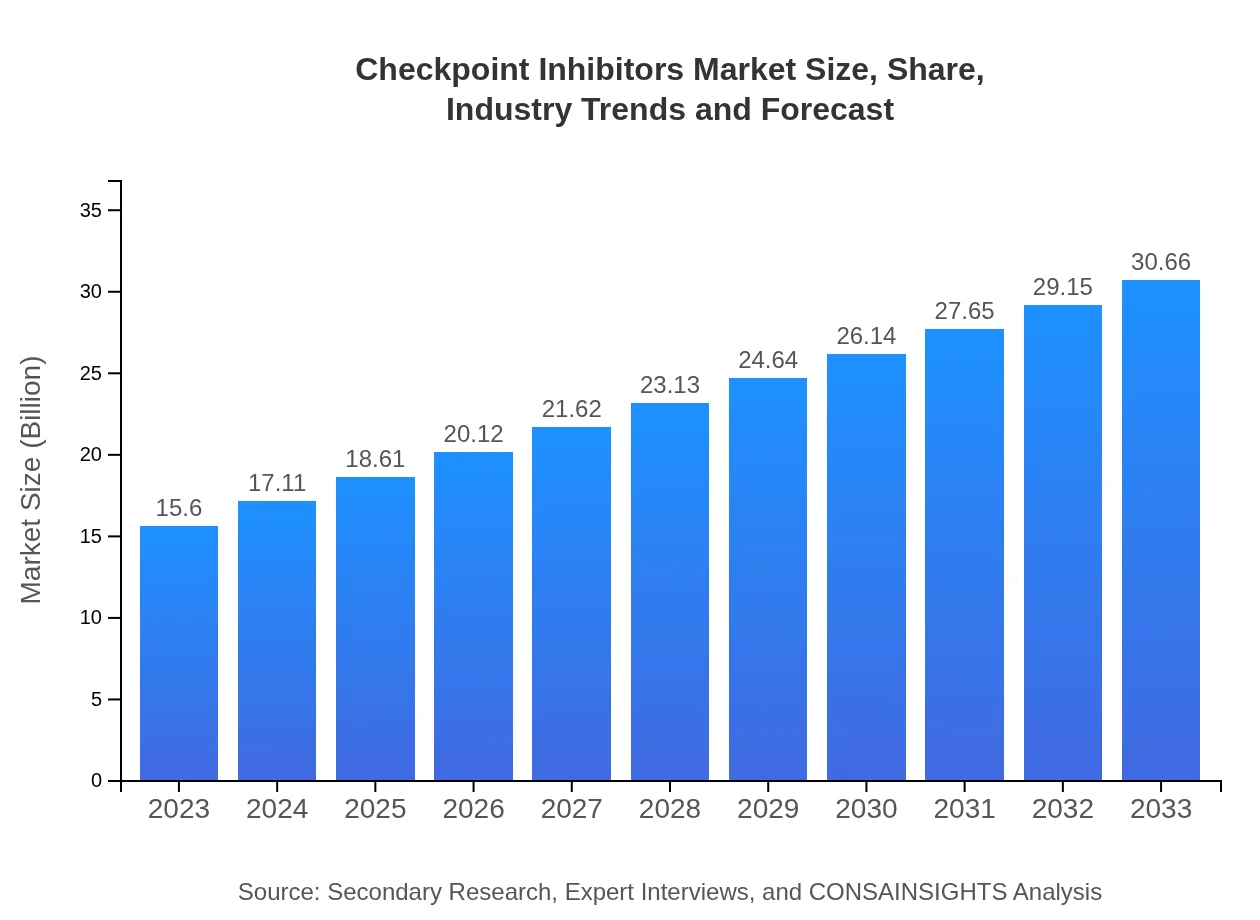
| 2023 Market Size | $15.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $30.66 Billion |
| Top Companies | Bristol-Myers Squibb, Roche, Merck & Co., AstraZeneca, Novartis |
| Last Modified Date | 31 January 2026 |
Checkpoint Inhibitors Market Overview
Customize Checkpoint Inhibitors Market Report market research report
- ✔ Get in-depth analysis of Checkpoint Inhibitors market size, growth, and forecasts.
- ✔ Understand Checkpoint Inhibitors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Checkpoint Inhibitors
What is the Market Size & CAGR of Checkpoint Inhibitors market in 2023?
Checkpoint Inhibitors Industry Analysis
Checkpoint Inhibitors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Checkpoint Inhibitors Market Analysis Report by Region
Europe Checkpoint Inhibitors Market Report:
The European Checkpoint Inhibitors market is projected to surge from $4.47 billion in 2023 to approximately $8.79 billion by 2033. Factors influencing this growth include robust healthcare systems, proactive regulatory environments, and a high prevalence of cancer across various European countries.Asia Pacific Checkpoint Inhibitors Market Report:
The Asia Pacific Checkpoint Inhibitors market is projected to grow from $2.97 billion in 2023 to $5.83 billion by 2033. The expansion is fueled by a surge in cancer cases, improved healthcare infrastructure, and growing awareness regarding immunotherapies. Collaborations among pharmaceutical firms and regional governments to increase R&D investments will enhance market growth.North America Checkpoint Inhibitors Market Report:
In North America, the market size is forecasted to increase from about $5.73 billion in 2023 to $11.27 billion by 2033. The U.S. dominates this region, largely due to high levels of investment in healthcare, significant innovations in drug formulations, and the presence of key industry players.South America Checkpoint Inhibitors Market Report:
The South American market is expected to grow from $1.31 billion in 2023 to $2.58 billion by 2033. Increased healthcare spending and the rising incidence of cancer in the region drive this growth. Improved regulatory frameworks and access to advanced therapies contribute significantly to the market's expansion.Middle East & Africa Checkpoint Inhibitors Market Report:
The Checkpoint Inhibitors market in the Middle East and Africa is expected to grow from $1.11 billion in 2023 to $2.19 billion in 2033. It is anticipated that the market will benefit from enhanced healthcare initiatives and the increasing prevalence of various cancers in the region.Tell us your focus area and get a customized research report.
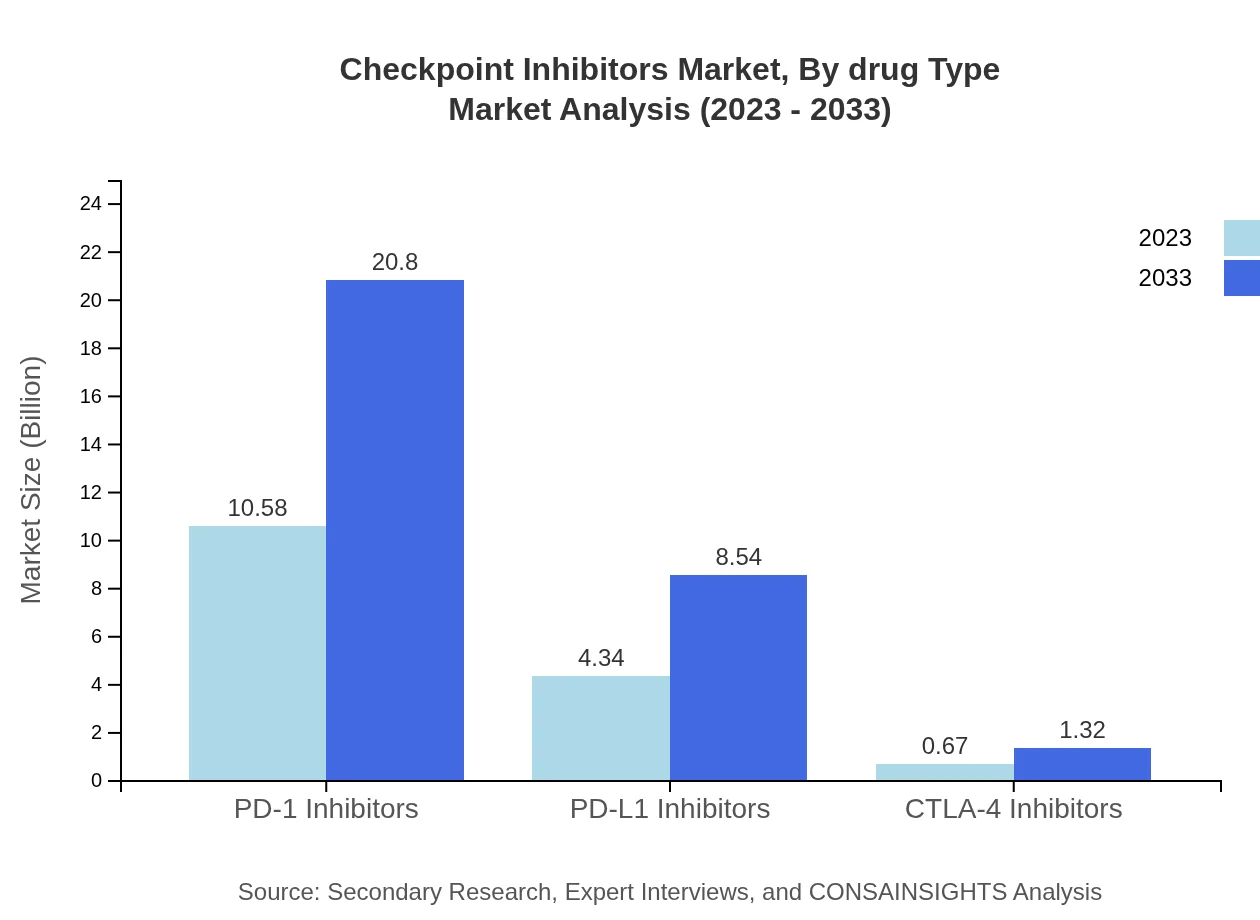
Checkpoint Inhibitors Market Analysis By Drug Type
The market breakdown by drug type indicates significant dominance of PD-1 inhibitors, showing growth from $10.58 billion in 2023 to $20.80 billion by 2033, capturing a 67.84% market share. PD-L1 inhibitors also demonstrate substantial growth, rising from $4.34 billion in 2023 to $8.54 billion by 2033, accounting for 27.84% share. CTLA-4 inhibitors, although smaller, will grow steadily from $0.67 billion to $1.32 billion, maintaining a 4.32% market share.
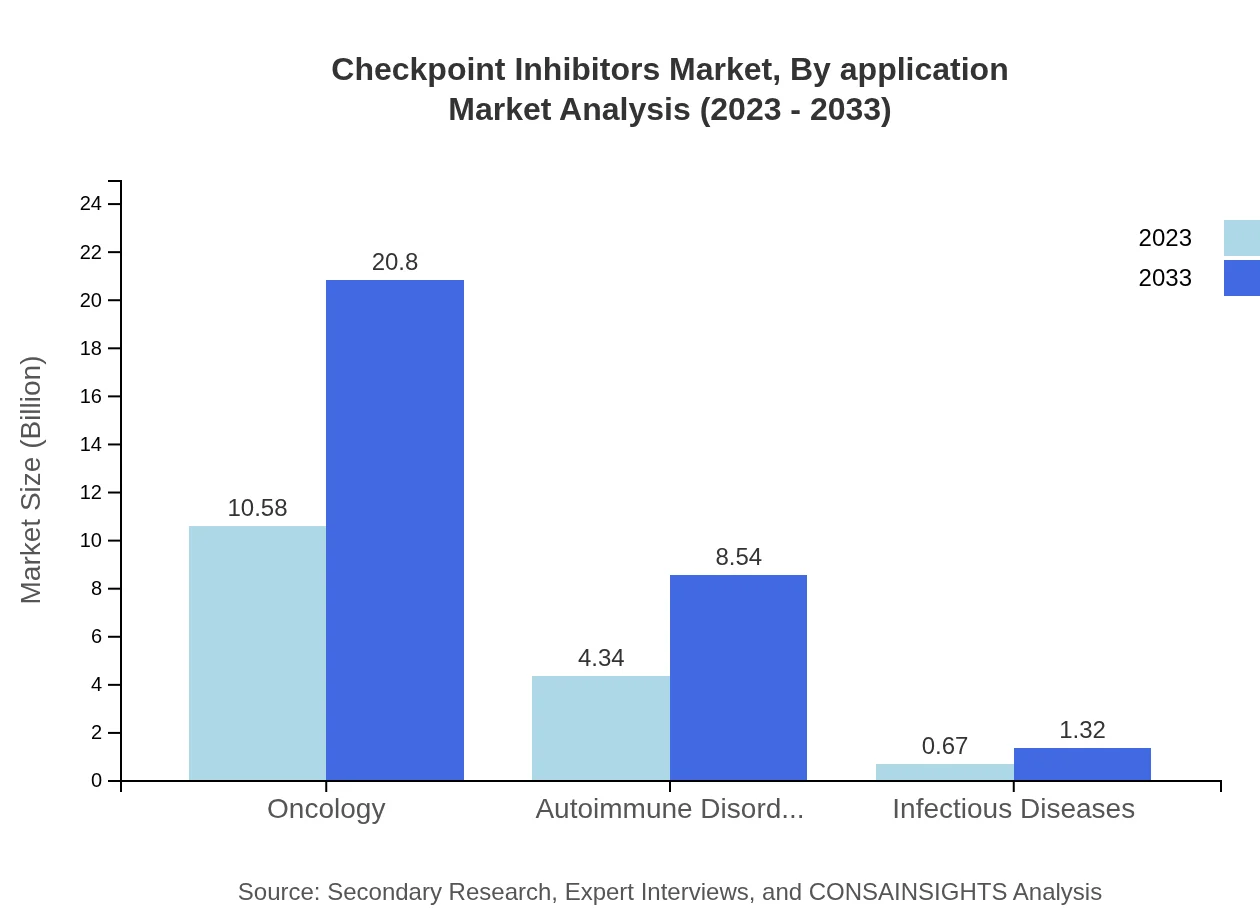
Checkpoint Inhibitors Market Analysis By Application
Oncology remains the leading application area for checkpoint inhibitors, with an anticipated growth from $10.58 billion in 2023 to $20.80 billion by 2033, solidifying its 67.84% share. Autoimmune disorders and infectious diseases, while growing from $4.34 billion and $0.67 billion respectively, will collectively hold significant market importance due to their rising diagnosis rates.
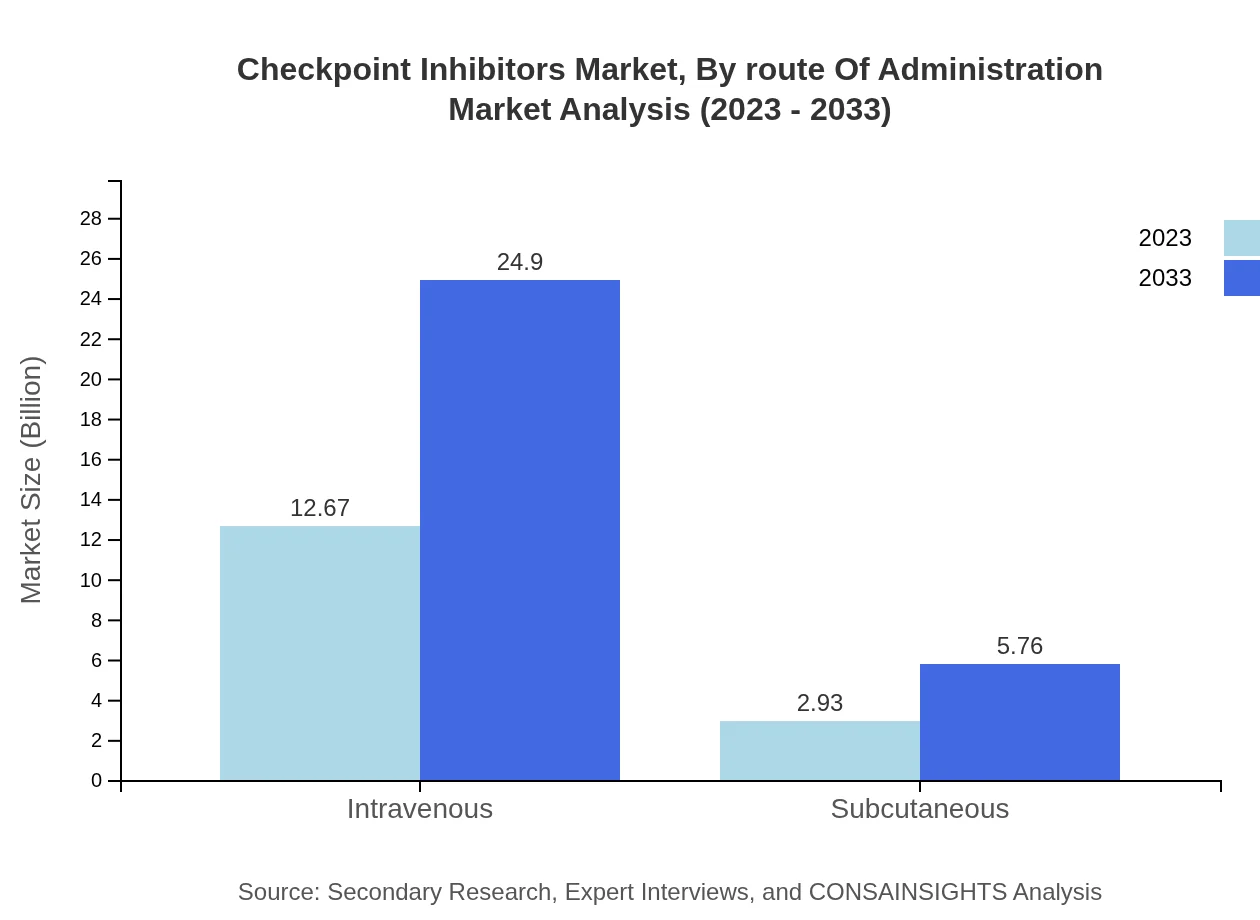
Checkpoint Inhibitors Market Analysis By Route Of Administration
The intravenous route dominates the administration landscape, forecasted to grow from $12.67 billion in 2023 to $24.90 billion by 2033, commanding an 81.2% market share. Subcutaneous administration shows a rising trend from $2.93 billion to $5.76 billion with a share of 18.8%, indicating a growing preference for less invasive administration techniques.
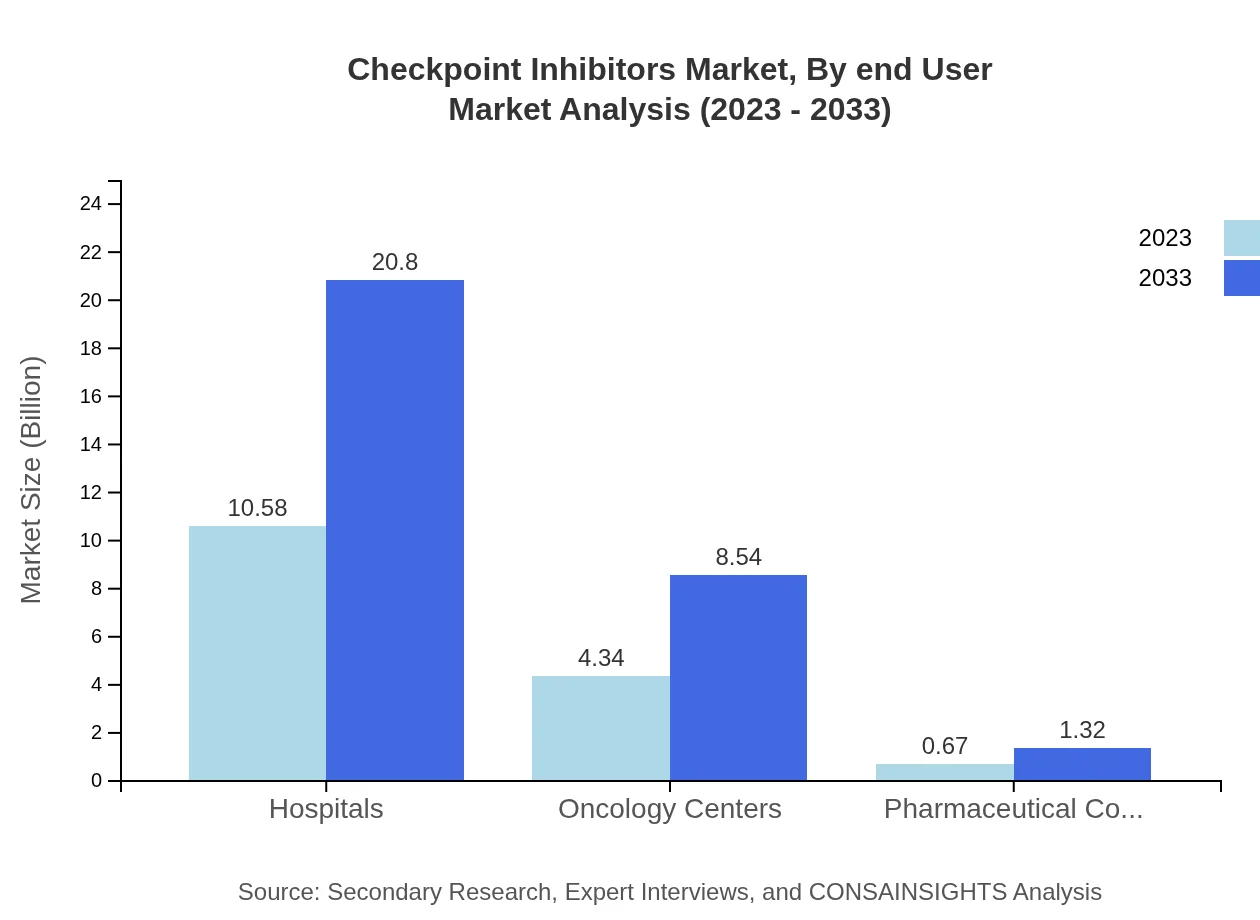
Checkpoint Inhibitors Market Analysis By End User
Hospitals represent the largest end-user segment, with expected growth from $10.58 billion in 2023 to $20.80 billion in 2033, retaining a dominant 67.84% market share. Oncology centers are also significant, growing from $4.34 billion to $8.54 billion in the same period, holding a 27.84% market share.
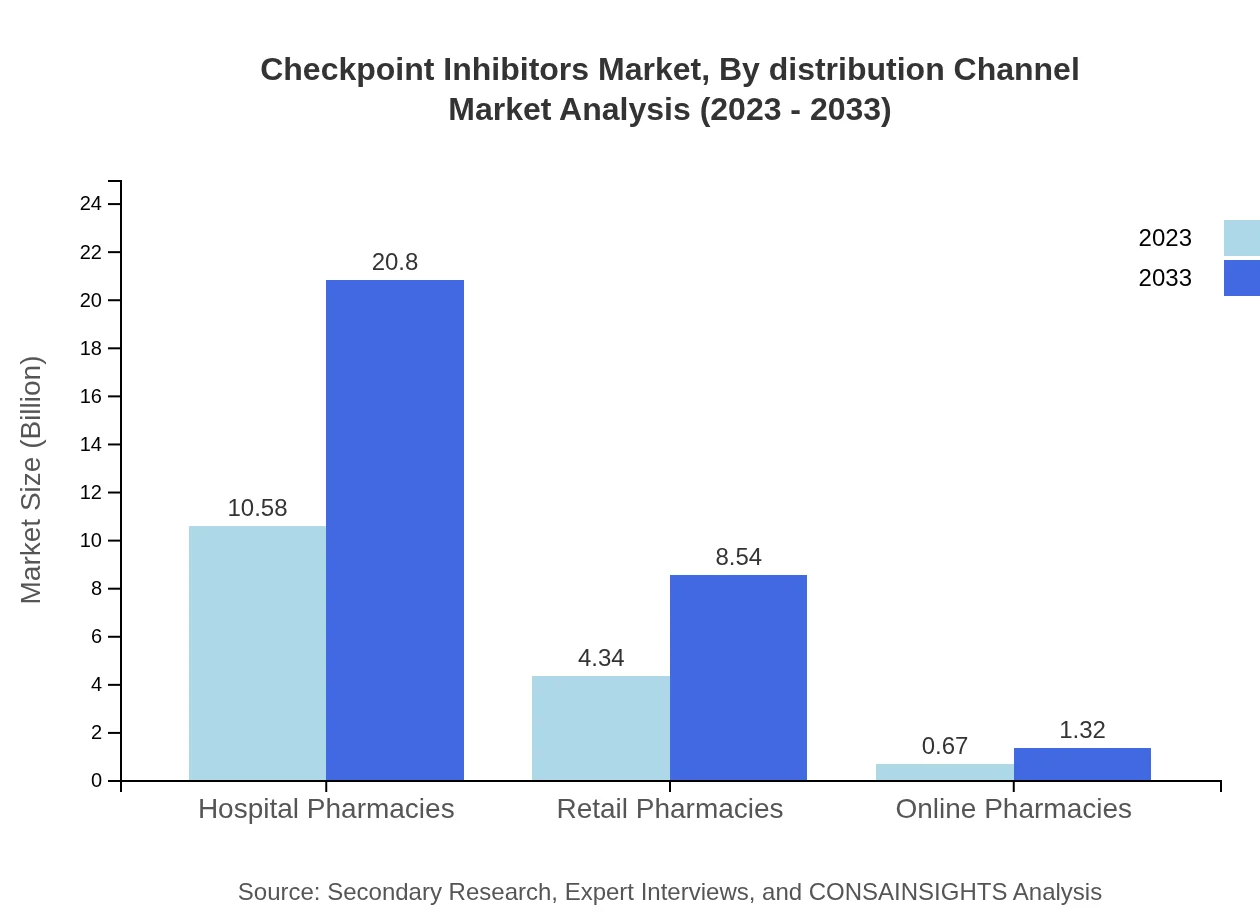
Checkpoint Inhibitors Market Analysis By Distribution Channel
The distribution channels encompass hospital pharmacies, retail pharmacies, and online pharmacies, where hospital pharmacies are projected to capture a sizable share, growing from $10.58 billion to $20.80 billion by 2033, commanding 67.84%. Retail and online pharmacies will grow steadily but account for a smaller market segment, emphasizing diverse access points for consumers.
Checkpoint Inhibitors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Checkpoint Inhibitors Industry
Bristol-Myers Squibb:
A pioneer in immunotherapy, Bristol-Myers Squibb developed Opdivo (nivolumab) and Yervoy (ipilimumab), leading products in the PD-1 and CTLA-4 inhibitor markets.Roche:
Roche's development of atezolizumab (Tecentriq) has set a benchmark in the PD-L1 inhibitor segment, contributing significantly to its strong position in the oncology market.Merck & Co.:
Known for Keytruda (pembrolizumab), Merck & Co. is a market leader in PD-1 inhibitors, with extensive data supporting its efficacy across multiple cancer types.AstraZeneca:
AstraZeneca's innovative approach with Imfinzi (durvalumab) positions it as a strong contender in the PD-L1 space, expanding options in cancer immunotherapy.Novartis:
With ongoing research into novel checkpoint inhibitors, Novartis continues to enhance its product portfolio and strengthens its market share in the oncology segment.We're grateful to work with incredible clients.









FAQs
What is the market size of checkpoint Inhibitors?
The checkpoint-inhibitors market is projected to reach approximately $15.6 billion by 2033, growing at a CAGR of 6.8% from a market size of $15.6 billion in 2023.
What are the key market players or companies in this checkpoint Inhibitors industry?
Key players in the checkpoint-inhibitors market include major pharmaceutical companies such as Bristol-Myers Squibb, Merck & Co., Roche, AstraZeneca, and Novartis, which focus on innovative therapies and research.
What are the primary factors driving the growth in the checkpoint Inhibitors industry?
Growth in the checkpoint-inhibitors market is driven by increasing incidence of cancer, advancements in biologics, growing research initiatives, and enhanced R&D investments by pharmaceutical companies.
Which region is the fastest Growing in the checkpoint Inhibitors?
The North America region is expected to witness significant growth in the checkpoint-inhibitors market, increasing from $5.73 billion in 2023 to $11.27 billion by 2033, driven by advanced healthcare infrastructure and research.
Does ConsaInsights provide customized market report data for the checkpoint Inhibitors industry?
Yes, ConsaInsights offers customized market report data tailored to specific business needs within the checkpoint-inhibitors industry, providing actionable insights and strategic analysis.
What deliverables can I expect from this checkpoint Inhibitors market research project?
Deliverables from the checkpoint-inhibitors market research project include comprehensive market analysis, competitive landscape, growth forecasts, regional insights, and tailored recommendations for strategic planning.
What are the market trends of checkpoint Inhibitors?
Market trends for checkpoint-inhibitors include increased adoption of immunotherapies, a focus on combination therapies, advancing clinical trials, and expanding applications beyond oncology into autoimmune disorders.