Chlamydia Infection Diagnostics And Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: chlamydia-infection-diagnostics-and-therapeutics
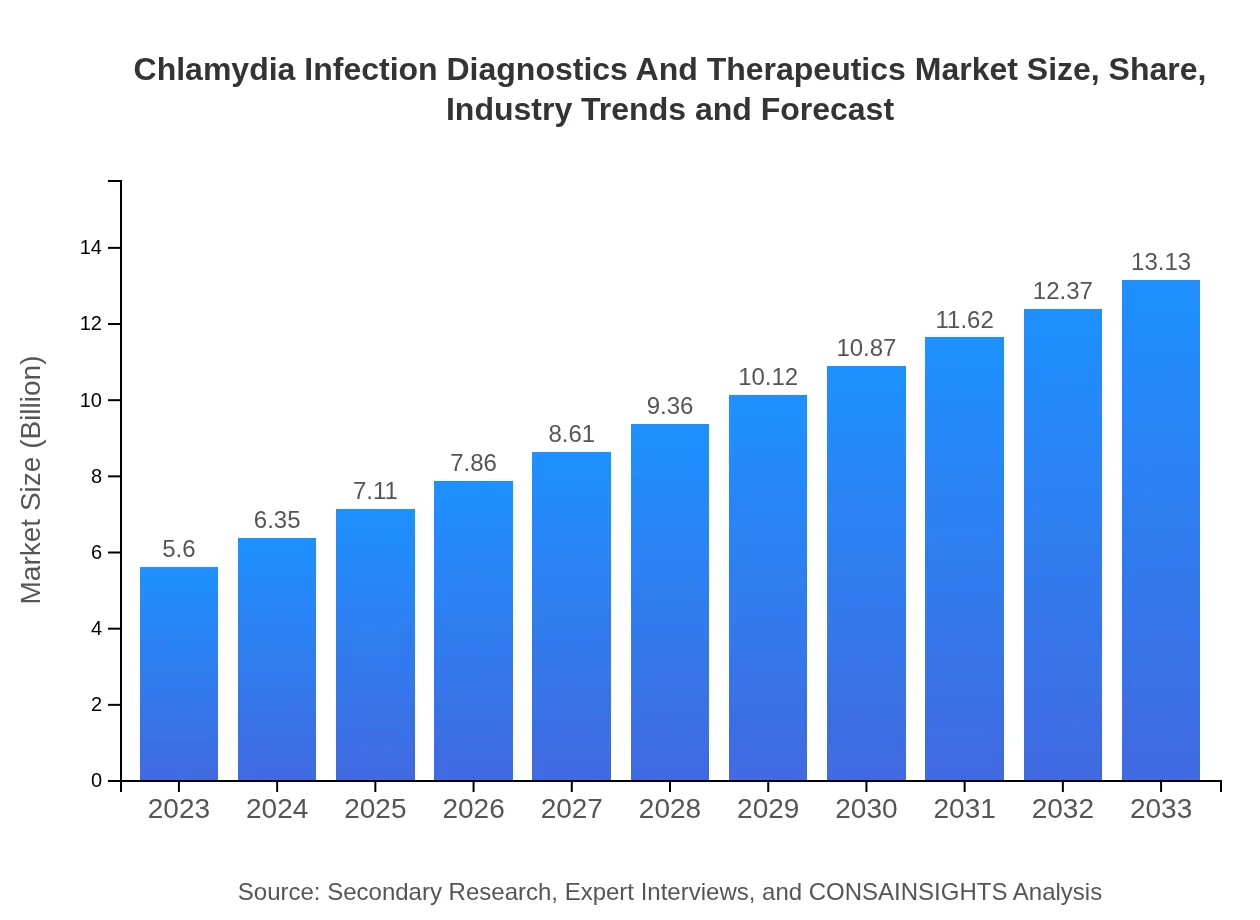
Chlamydia Infection Diagnostics And Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Chlamydia Infection Diagnostics and Therapeutics market, encompassing its size, growth forecasts from 2023 to 2033, and insights into current market dynamics and future trends.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 8.6% |
| 2033 Market Size | $13.13 Billion |
| Top Companies | Abbott Laboratories, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Hologic, Inc. |
| Last Modified Date | 31 January 2026 |
Chlamydia Infection Diagnostics And Therapeutics Market Overview
Customize Chlamydia Infection Diagnostics And Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Chlamydia Infection Diagnostics And Therapeutics market size, growth, and forecasts.
- ✔ Understand Chlamydia Infection Diagnostics And Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Chlamydia Infection Diagnostics And Therapeutics
What is the Market Size & CAGR of Chlamydia Infection Diagnostics And Therapeutics market in 2033?
Chlamydia Infection Diagnostics And Therapeutics Industry Analysis
Chlamydia Infection Diagnostics And Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
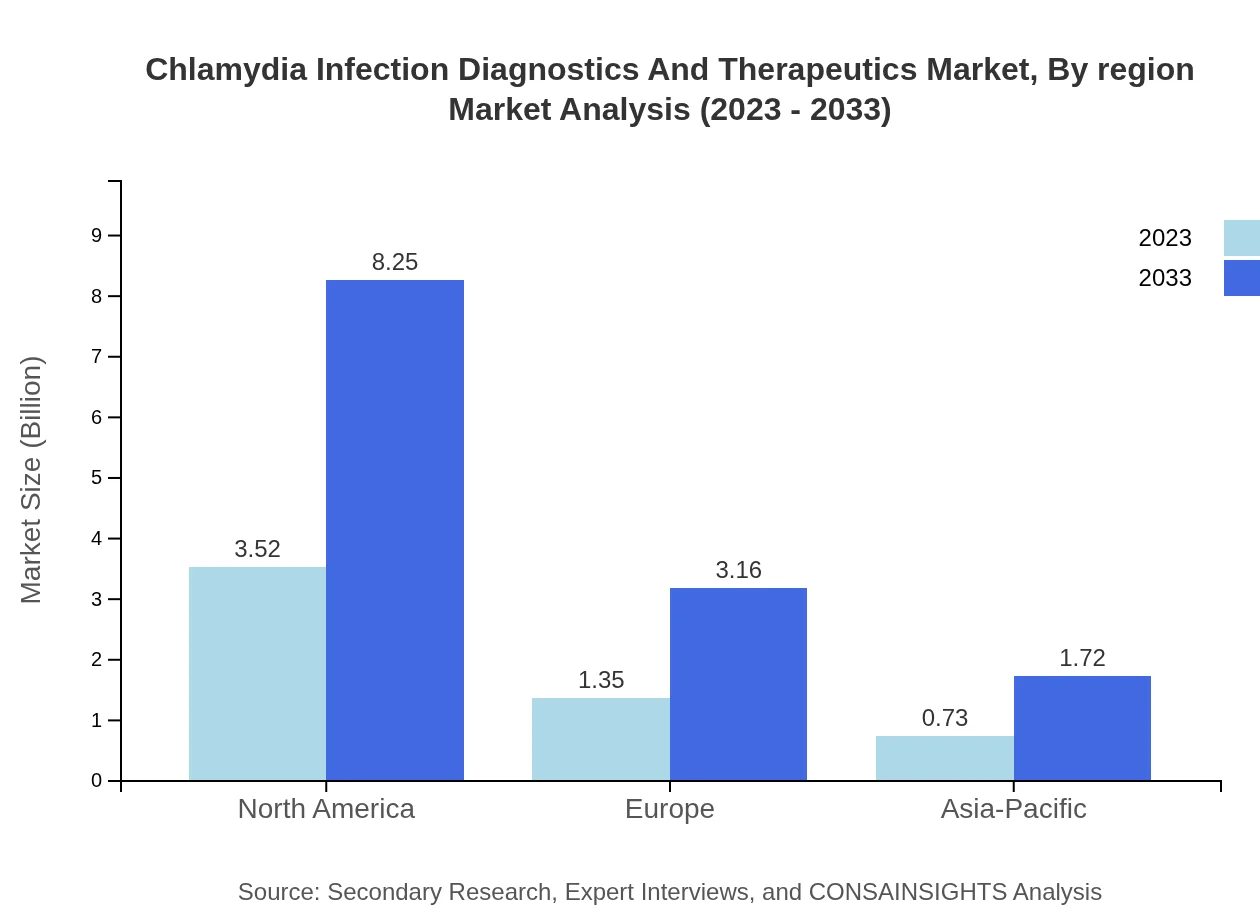
Chlamydia Infection Diagnostics And Therapeutics Market Analysis Report by Region
Europe Chlamydia Infection Diagnostics And Therapeutics Market Report:
The European market is anticipated to grow from USD 1.69 billion in 2023 to USD 3.97 billion in 2033, bolstered by stringent regulations on STI management and a focus on enhancing healthcare access.Asia Pacific Chlamydia Infection Diagnostics And Therapeutics Market Report:
The Asia Pacific market, valued at USD 1.11 billion in 2023, is projected to grow to USD 2.59 billion by 2033, driven by rising healthcare expenditures, the proliferation of advanced diagnostic technologies, and government initiatives to control STIs.North America Chlamydia Infection Diagnostics And Therapeutics Market Report:
North America is currently the largest market, with a value of USD 1.95 billion in 2023 and a projected growth to USD 4.56 billion by 2033. The region's strong emphasis on public health initiatives, high screening rates, and innovative diagnostic solutions are pivotal to this expansion.South America Chlamydia Infection Diagnostics And Therapeutics Market Report:
In South America, the market is expected to grow from USD 0.42 billion in 2023 to USD 0.98 billion in 2033. Factors contributing to this growth include increasing awareness of chlamydia infections and improvements in healthcare infrastructure.Middle East & Africa Chlamydia Infection Diagnostics And Therapeutics Market Report:
The Middle East and Africa market is projected to grow from USD 0.44 billion in 2023 to USD 1.02 billion by 2033. Growth is driven by rising awareness of sexual health and increased access to healthcare services.Tell us your focus area and get a customized research report.
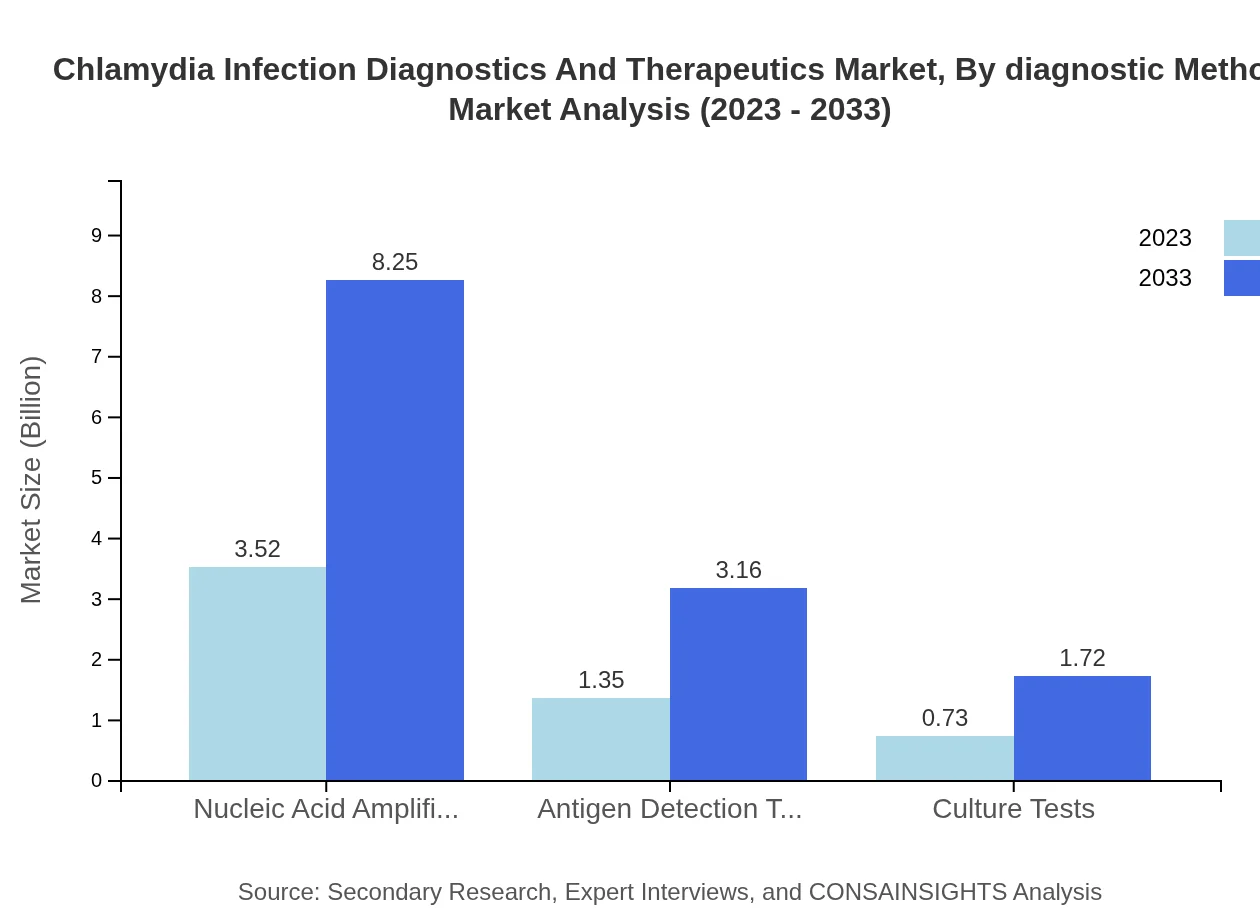
Chlamydia Infection Diagnostics And Therapeutics Market Analysis By Diagnostic Method
In the diagnostics segment, Nucleic Acid Amplification Tests (NAATs) dominate, projected to grow from USD 3.52 billion in 2023 to USD 8.25 billion by 2033, representing 62.86% market share. Antigen Detection Tests and Culture Tests follow, increasing in value as demand shifts towards fast and reliable testing.
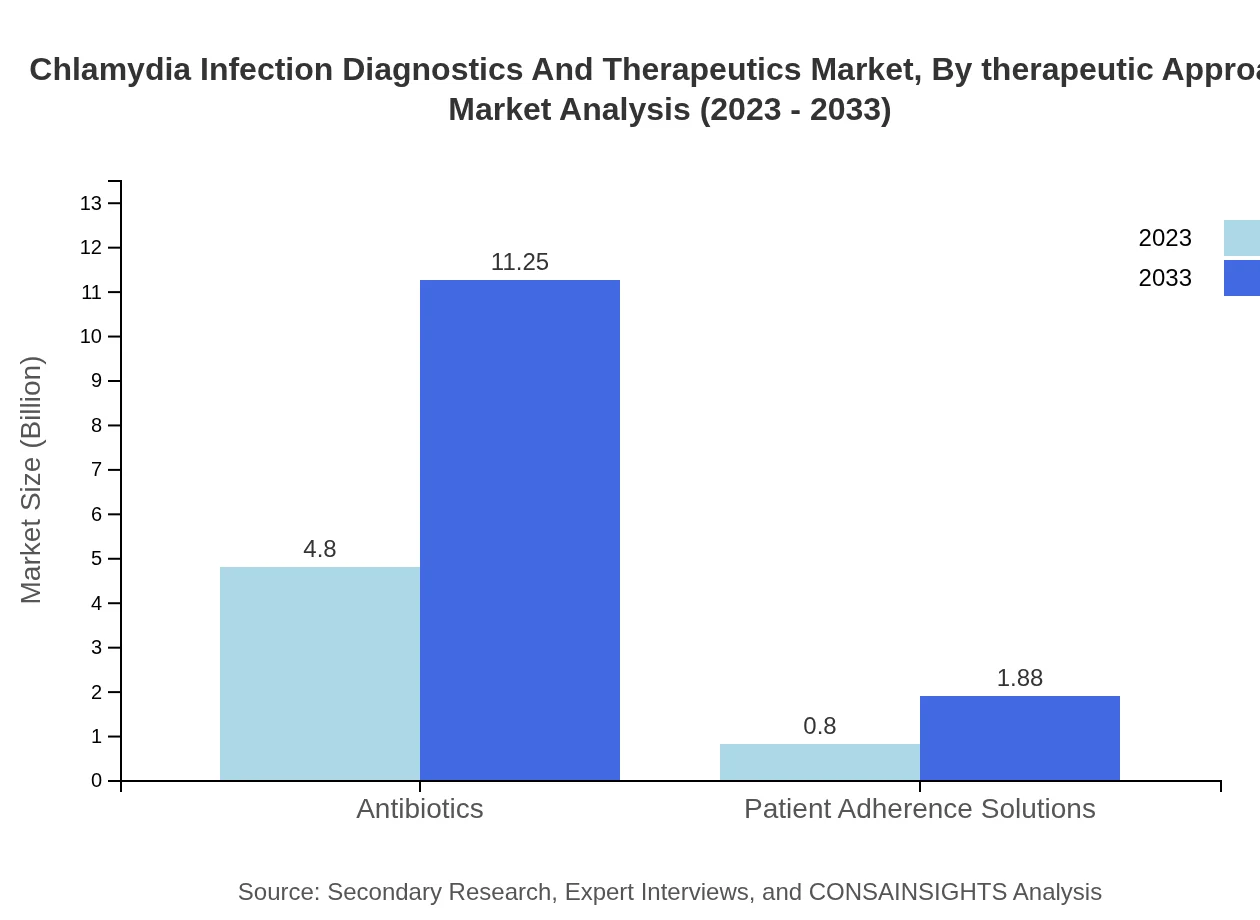
Chlamydia Infection Diagnostics And Therapeutics Market Analysis By Therapeutic Approach
Antibiotics remain the cornerstone of chlamydia treatment, expected to reach USD 11.25 billion by 2033, holding 85.7% market share. Patient adherence solutions will also see growth, moving from USD 0.80 billion in 2023 to USD 1.88 billion by 2033.
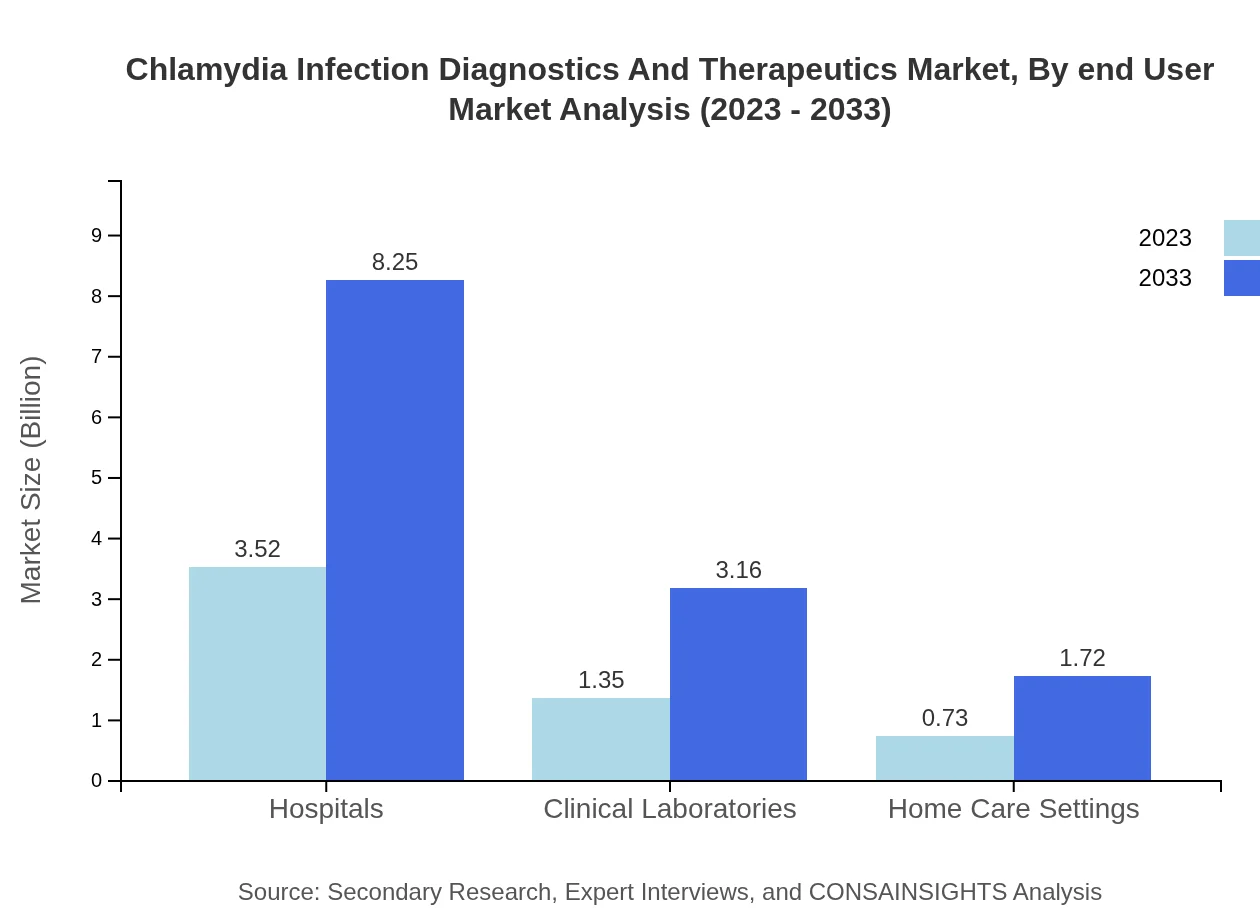
Chlamydia Infection Diagnostics And Therapeutics Market Analysis By End User
Hospitals represent the largest end-user segment, projected to grow from USD 3.52 billion in 2023 to USD 8.25 billion by 2033, retaining a 62.86% share. Clinical laboratories and home care settings will also expand their market penetration significantly.
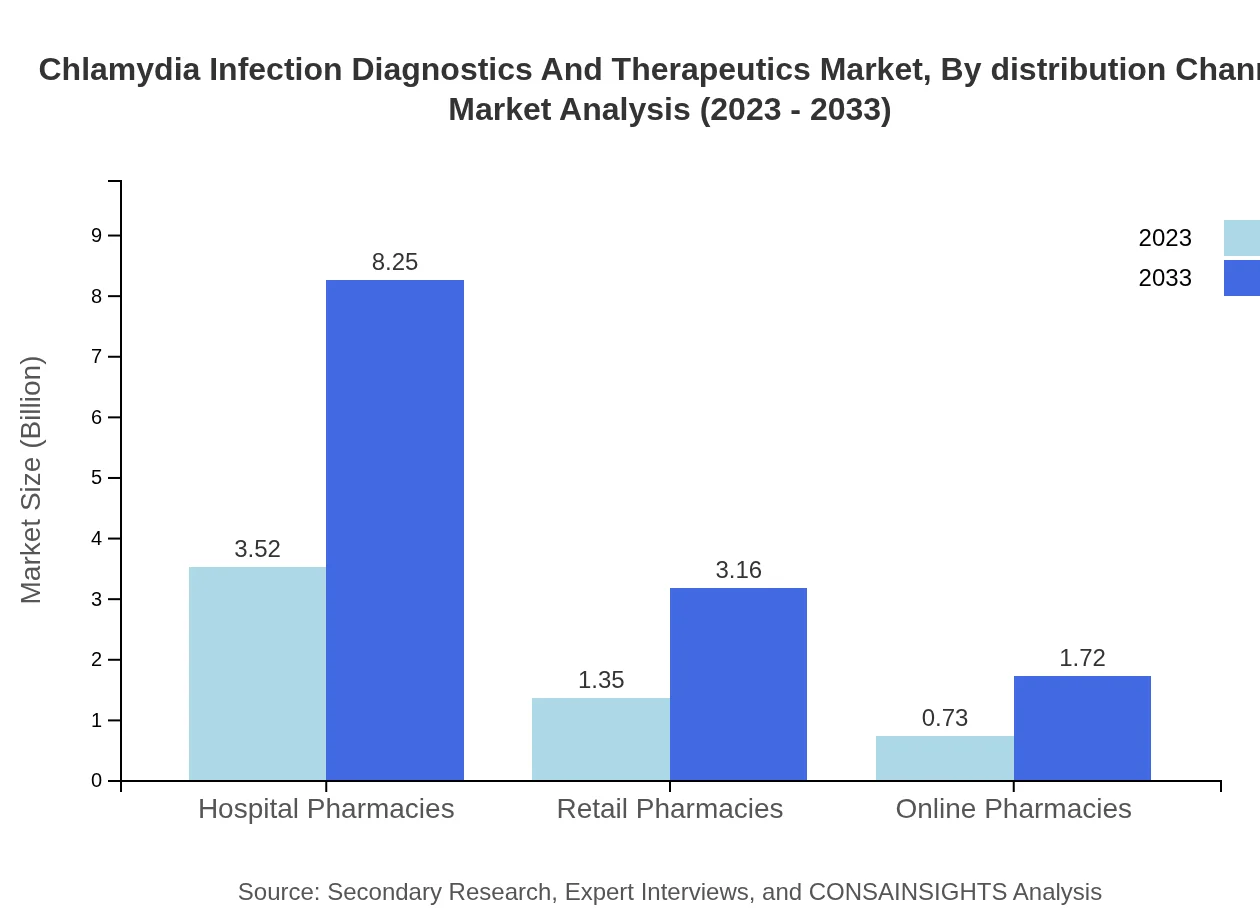
Chlamydia Infection Diagnostics And Therapeutics Market Analysis By Distribution Channel
Hospital pharmacies are anticipated to dominate the distribution channel segment, with a market size of USD 3.52 billion in 2023 and expected growth to USD 8.25 billion by 2033. Retail and online pharmacies are also expanding rapidly in response to increased consumer demand.
Chlamydia Infection Diagnostics And Therapeutics Market Analysis By Region
Regional analysis indicates North America as the largest market, followed by Europe and Asia Pacific. Variances in growth rates reflect local healthcare policies, disease awareness levels, and technological advances in diagnostics.
Chlamydia Infection Diagnostics And Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Chlamydia Infection Diagnostics And Therapeutics Industry
Abbott Laboratories:
A global healthcare company known for its innovative diagnostic products, including NAATs for detecting chlamydia.F. Hoffmann-La Roche Ltd.:
A leading pharmaceutical and diagnostics company, Roche focuses on developing advanced tests and treatments for STIs.Thermo Fisher Scientific Inc.:
Provides laboratory equipment and diagnostic solutions, enhancing the accuracy and speed of chlamydia testing.Hologic, Inc.:
Specializes in women's health diagnostics, including products for chlamydia detection and treatment.We're grateful to work with incredible clients.









FAQs
What is the market size of chlamydia Infection Diagnostics And Therapeutics?
The global chlamydia infection diagnostics and therapeutics market is projected to reach approximately $5.6 billion by 2033, expanding at a CAGR of 8.6% from its current size, reflecting significant growth driven by advancements in diagnostic technologies and therapeutics.
What are the key market players or companies in the chlamydia Infection Diagnostics And Therapeutics industry?
Key players in the chlamydia infection diagnostics and therapeutics market include major pharmaceutical companies and diagnostic device manufacturers, potentially including Roche Diagnostics, Abbott Laboratories, Cepheid, and Hologic, all of which are integral in advancing product offerings.
What are the primary factors driving the growth in the chlamydia Infection Diagnostics And Therapeutics industry?
Growth in the industry is driven by increasing prevalence of chlamydia infections, rising awareness about sexually transmitted diseases, advancements in diagnostic technology, and growth in healthcare spending, all contributing to an expanded need for these diagnostics and therapeutics.
Which region is the fastest Growing in the chlamydia Infection Diagnostics And Therapeutics?
The fastest-growing region in the chlamydia infection diagnostics and therapeutics market is North America, projected to grow from $1.95 billion in 2023 to $4.56 billion by 2033, fueled by high healthcare expenditure and advanced healthcare infrastructure.
Does ConsaInsights provide customized market report data for the chlamydia Infection Diagnostics And Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to the needs of clients in the chlamydia infection diagnostics and therapeutics industry, ensuring relevant and actionable insights for strategic decision-making.
What deliverables can I expect from this chlamydia Infection Diagnostics And Therapeutics market research project?
Deliverables include a comprehensive market analysis report, data on market size and growth projections, competitive landscape insights, regional analysis, key trends, and segment-specific information designed to support your business strategy.
What are the market trends of chlamydia Infection Diagnostics And Therapeutics?
Current market trends include a shift towards home-based testing, integration of digital health solutions, increased focus on preventive healthcare, and emerging trends in personalized medicine, all defining the future landscape of diagnostics and therapeutics.