Clinical Chemistry Analyzers Market Report
Published Date: 31 January 2026 | Report Code: clinical-chemistry-analyzers
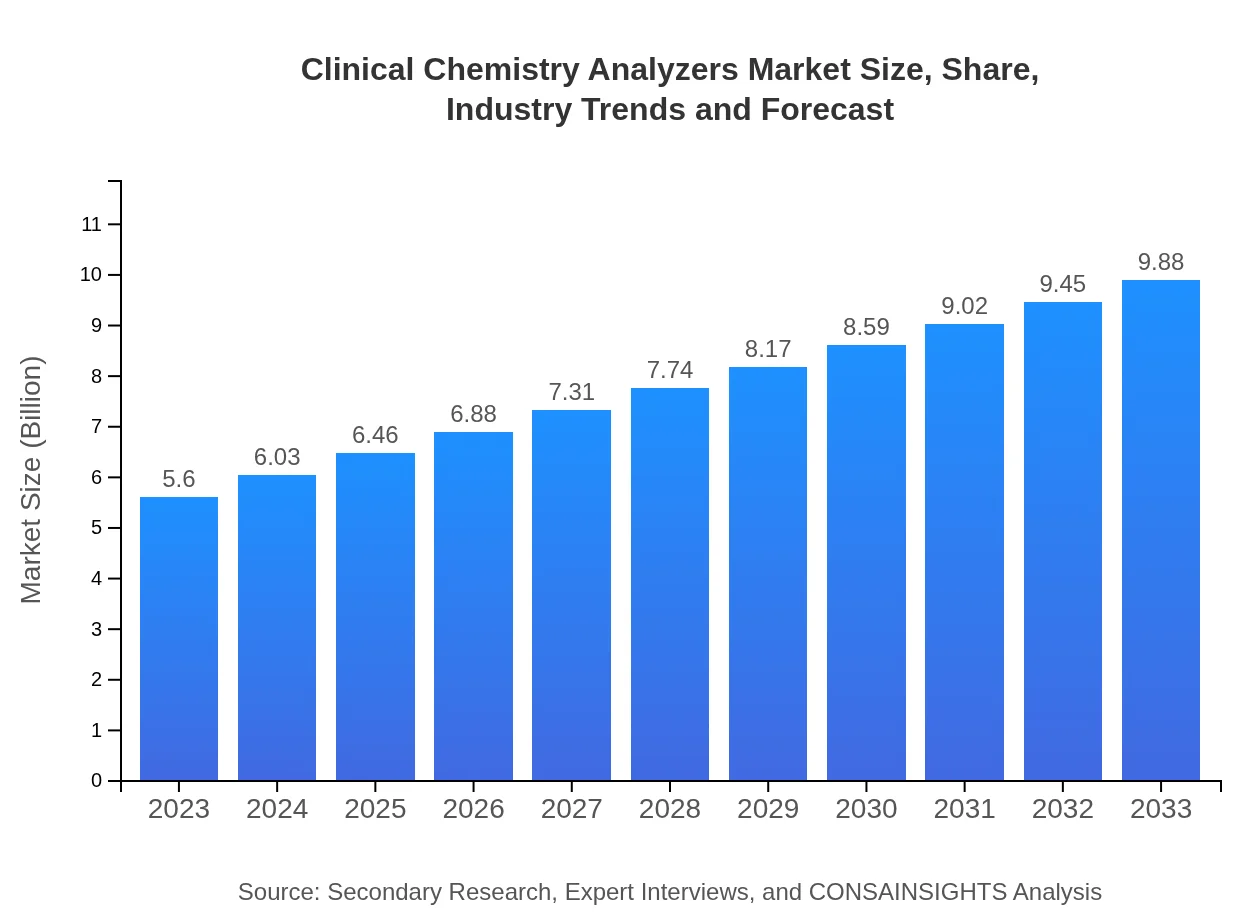
Clinical Chemistry Analyzers Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Clinical Chemistry Analyzers market, exploring its size, growth trends, segmentation, and regional dynamics, along with insights into leading companies and future market forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $9.88 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Clinical Chemistry Analyzers Market Overview
Customize Clinical Chemistry Analyzers Market Report market research report
- ✔ Get in-depth analysis of Clinical Chemistry Analyzers market size, growth, and forecasts.
- ✔ Understand Clinical Chemistry Analyzers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clinical Chemistry Analyzers
What is the Market Size & CAGR of Clinical Chemistry Analyzers market in 2023?
Clinical Chemistry Analyzers Industry Analysis
Clinical Chemistry Analyzers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clinical Chemistry Analyzers Market Analysis Report by Region
Europe Clinical Chemistry Analyzers Market Report:
In Europe, the market size for Clinical Chemistry Analyzers is projected to grow from 1.85 billion USD in 2023 to approximately 3.27 billion USD by 2033. An aging population, along with rising incidences of chronic illnesses, drives demand for advanced diagnostic solutions. Additionally, European countries' investment in healthcare infrastructure and technology adoption plays a crucial role in enhancing market growth.Asia Pacific Clinical Chemistry Analyzers Market Report:
In the Asia Pacific region, the Clinical Chemistry Analyzers market was valued at approximately 0.98 billion USD in 2023 and is expected to reach 1.73 billion USD by 2033. The growth is facilitated by increased healthcare investments, rising prevalence of chronic diseases, and expanding healthcare infrastructure. Additionally, the adoption of advanced diagnostics in countries like China and India is significantly contributing to market expansion.North America Clinical Chemistry Analyzers Market Report:
North America holds a significant share of the Clinical Chemistry Analyzers market, with an estimated value of 2.06 billion USD in 2023, anticipated to reach about 3.63 billion USD by 2033. Factors contributing to this growth include a well-established healthcare system, ongoing innovations in medical technology, and increasing demand for efficient diagnostic tools. The region's emphasis on early disease detection and preventive healthcare is heightening the reliance on clinical chemistry diagnostics.South America Clinical Chemistry Analyzers Market Report:
The South American market for Clinical Chemistry Analyzers had a value of 0.44 billion USD in 2023, projected to rise to 0.78 billion USD by 2033. The escalating demand for diagnostic services, coupled with an increase in healthcare spending, are vital drivers for this market. Moreover, the focus on improving clinical laboratory services and bolstering disease control strategies in the region is further accelerating growth.Middle East & Africa Clinical Chemistry Analyzers Market Report:
The Clinical Chemistry Analyzers market in the Middle East and Africa is valued at 0.26 billion USD in 2023, expected to grow to 0.47 billion USD by 2033. Increasing healthcare expenditure, a focus on improving healthcare quality, and the expansion of laboratory networks are essential for this region's market growth. The rising demand for efficient healthcare services is inadvertently boosting the adoption of advanced clinical chemistry analysis technologies.Tell us your focus area and get a customized research report.
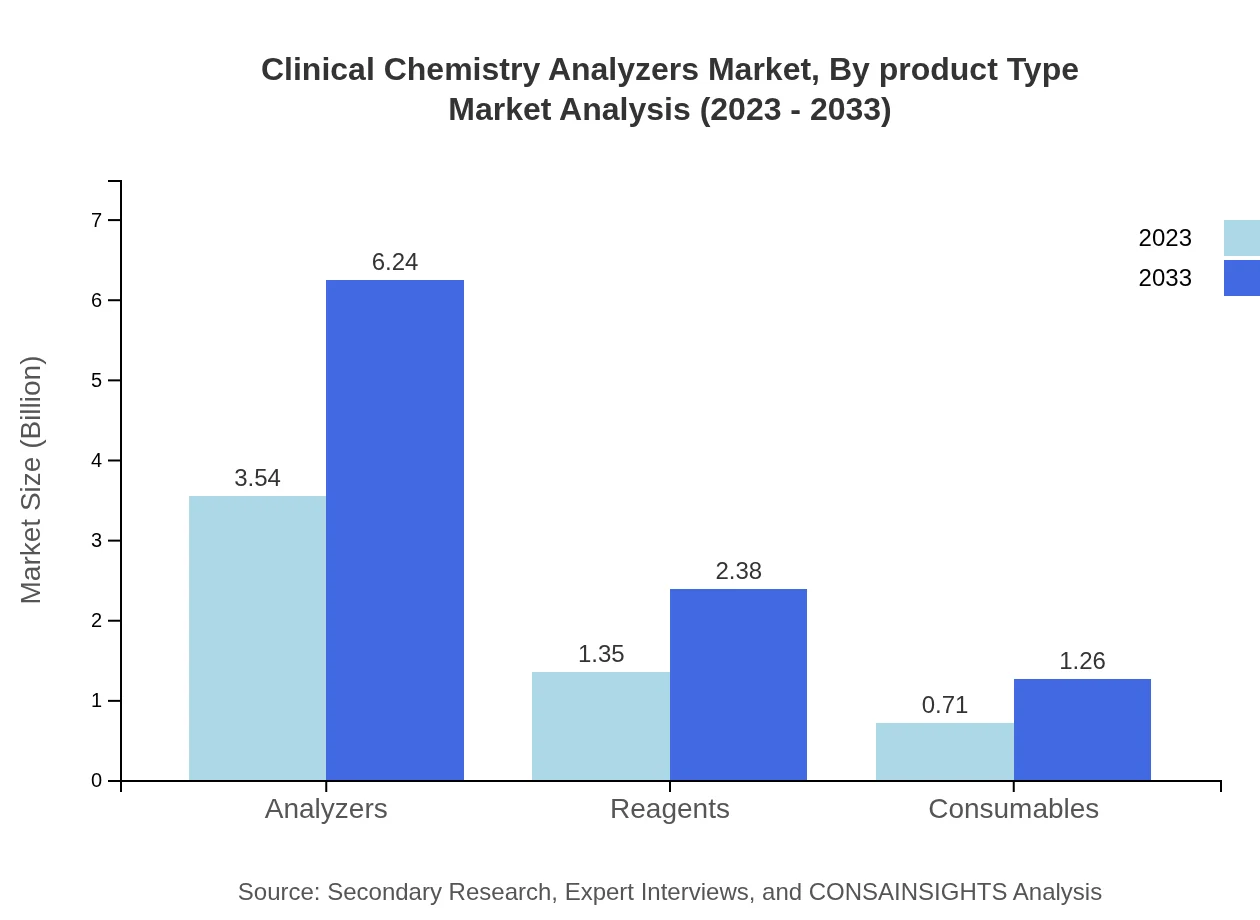
Clinical Chemistry Analyzers Market Analysis By Product Type
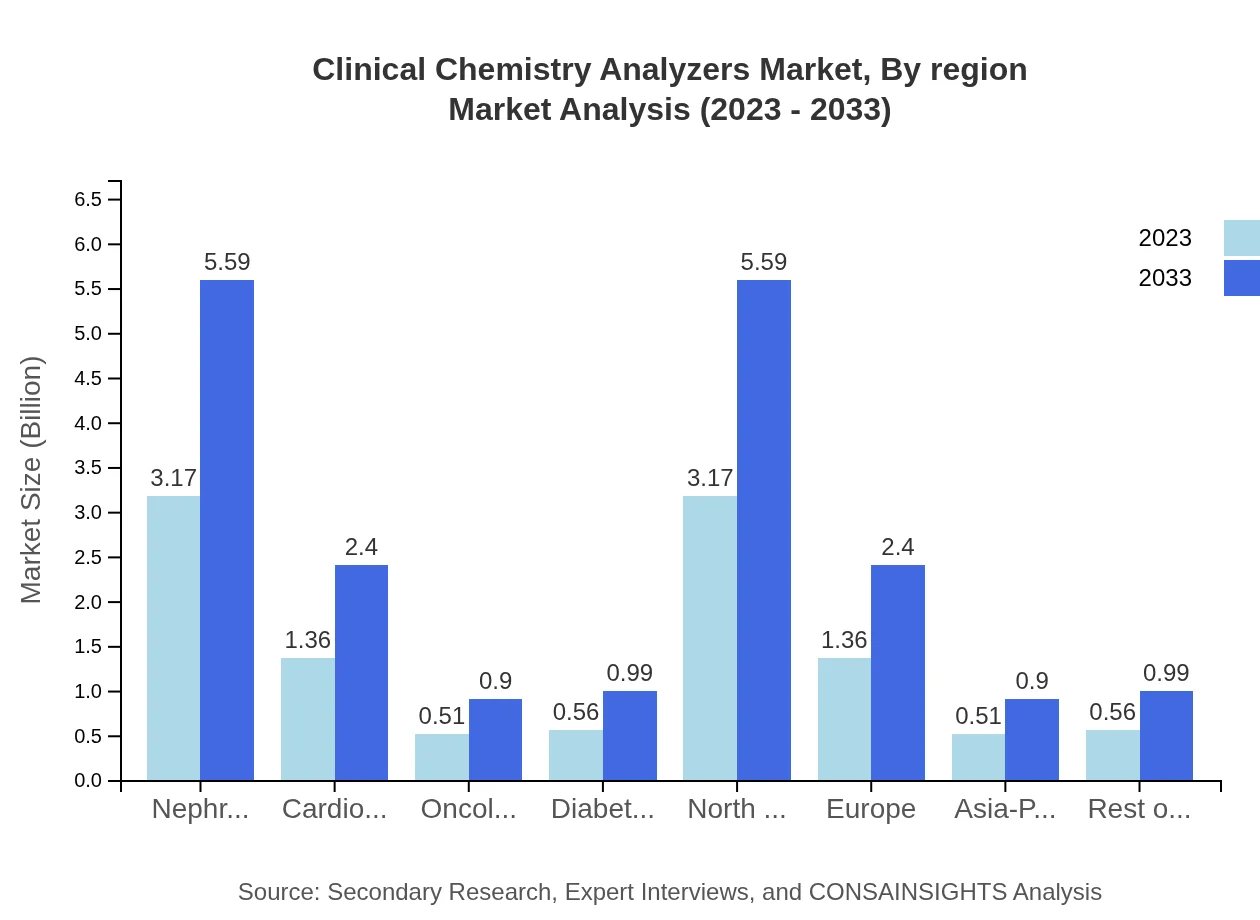
In terms of product type, the Clinical Chemistry Analyzers market showcases significant growth in the Nephrology segment, valued at 3.17 billion USD in 2023 and predicted to reach 5.59 billion USD by 2033, holding a consistent market share of 56.62%. The Cardiology segment, worth 1.36 billion USD in 2023, is projected to grow to 2.40 billion USD by 2033, capturing 24.33% market share. The Oncology segment, valued at 0.51 billion USD in 2023, will expand to 0.90 billion USD by 2033, representing 9.07% of the market. Moreover, the Diabetes segment is estimated to range from 0.56 billion USD (2023) to 0.99 billion USD (2033), reflecting 9.98% of the market share.
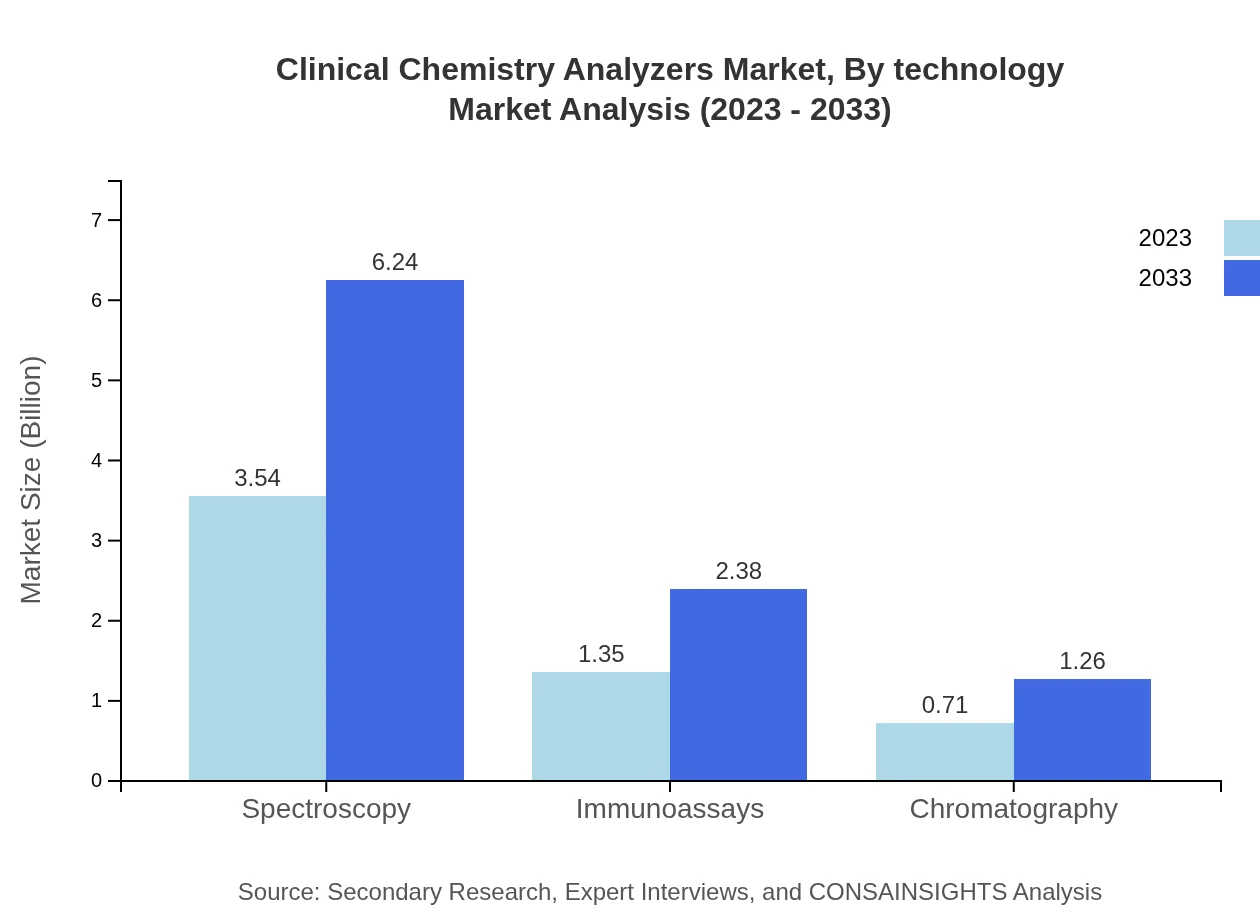
Clinical Chemistry Analyzers Market Analysis By Technology
Analyzers using spectroscopy technology dominate the market, with a share of 63.21%, growing from 3.54 billion USD in 2023 to 6.24 billion USD by 2033. The immunoassays segment follows, valued at 1.35 billion USD in 2023 and projected to shift to 2.38 billion USD by 2033 with a 24.05% market share. Chromatography, currently at 0.71 billion USD, anticipates reaching 1.26 billion USD by 2033, possessing a 12.74% share.
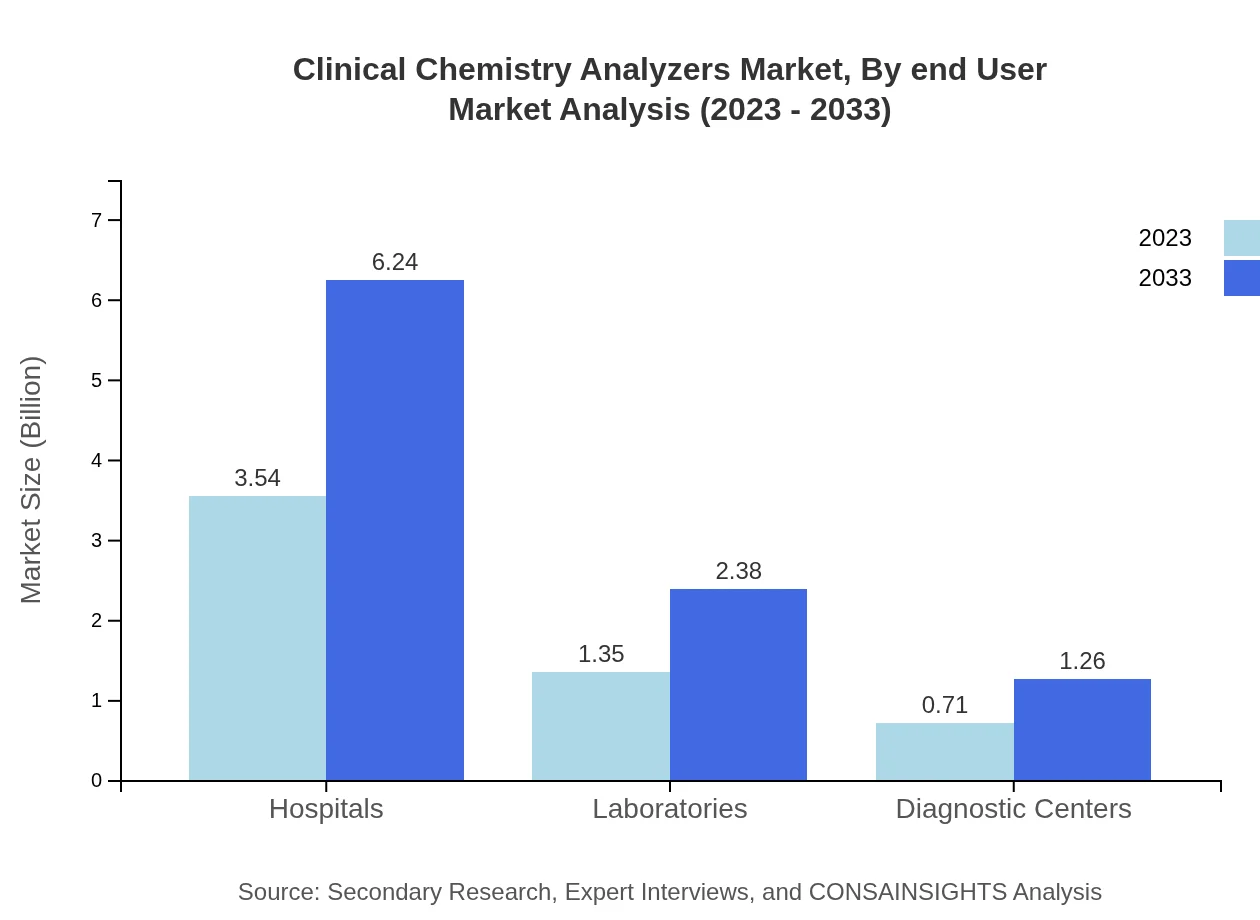
Clinical Chemistry Analyzers Market Analysis By End User
The clinical chemistry analyzers market by end-user shows hospitals leading with a share of 63.21%, growing from 3.54 billion USD in 2023 to 6.24 billion USD by 2033. Laboratories hold 24.05% of the market, starting at 1.35 billion USD in 2023 and climbing to 2.38 billion USD by 2033. Diagnostic centers are expanding from 0.71 billion USD (2023) to 1.26 billion USD (2033) with a 12.74% market share.
Clinical Chemistry Analyzers Market Analysis By Region
The Clinical Chemistry Analyzers market shows regional variations, with North America leading in size, followed by Europe. The Asia Pacific market is rapidly growing due to increased urbanization and healthcare investments. South America and Middle East/Africa are also expected to contribute with modest growth rates through improved healthcare access and technological integration in diagnostics.
Clinical Chemistry Analyzers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clinical Chemistry Analyzers Industry
Roche Diagnostics:
A leading player in the diagnostics field, Roche offers an extensive range of clinical chemistry analyzers known for their innovative technology and efficiency in various laboratory settings.Abbott Laboratories:
Abbott is recognized for its pioneering work in medical diagnostics, providing advanced clinical chemistry analyzers that support healthcare professionals in delivering high-quality patient care.Siemens Healthineers:
With a strong focus on diagnostic imaging and laboratory diagnostics, Siemens provides wide-ranging clinical chemistry analyzers designed to meet the evolving needs of laboratories.Thermo Fisher Scientific:
A leading name in scientific equipment, Thermo Fisher offers a diverse portfolio of clinical chemistry analyzers, integrating cutting-edge technology to enhance diagnostic accuracy.We're grateful to work with incredible clients.









FAQs
What is the market size of clinical Chemistry Analyzers?
The market size of clinical chemistry analyzers is approximately $5.6 billion in 2023, with a projected CAGR of 5.7%. By 2033, the market is expected to see significant growth, reflecting increasing demand and advancements in technology.
What are the key market players or companies in the clinical Chemistry Analyzers industry?
Key players in the clinical chemistry analyzers industry include Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Beckman Coulter, and Thermo Fisher Scientific, among others. These companies significantly influence the market through innovation and product development.
What are the primary factors driving the growth in the clinical Chemistry Analyzers industry?
Growth in the clinical chemistry analyzers industry is driven by factors such as the rising prevalence of chronic diseases, increasing demand for accurate diagnostic tests, advancements in technology, and the need for rapid testing in clinical settings.
Which region is the fastest Growing in the clinical Chemistry Analyzers?
Among various regions, Europe is the fastest-growing market for clinical chemistry analyzers, projected to rise from $1.85 billion in 2023 to $3.27 billion by 2033. This growth is attributed to enhanced healthcare infrastructure and increased investment in medical technologies.
Does ConsaInsights provide customized market report data for the clinical Chemistry Analyzers industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the clinical chemistry analyzers industry. This includes detailed analysis of market trends, competitor insights, and regional variations to facilitate informed decision-making.
What deliverables can I expect from this clinical Chemistry Analyzers market research project?
Deliverables from the clinical chemistry analyzers market research project include comprehensive market analysis, segment data, regional insights, competitive landscape assessment, and actionable recommendations to guide business strategies in this sector.
What are the market trends of clinical Chemistry Analyzers?
Current market trends in clinical chemistry analyzers indicate an upward trajectory due to technological advancements, an increase in point-of-care testing, and a shift towards automation, ensuring efficient and accurate diagnostic processes.