Clinical Immunoassay Analyzers Market Report
Published Date: 31 January 2026 | Report Code: clinical-immunoassay-analyzers
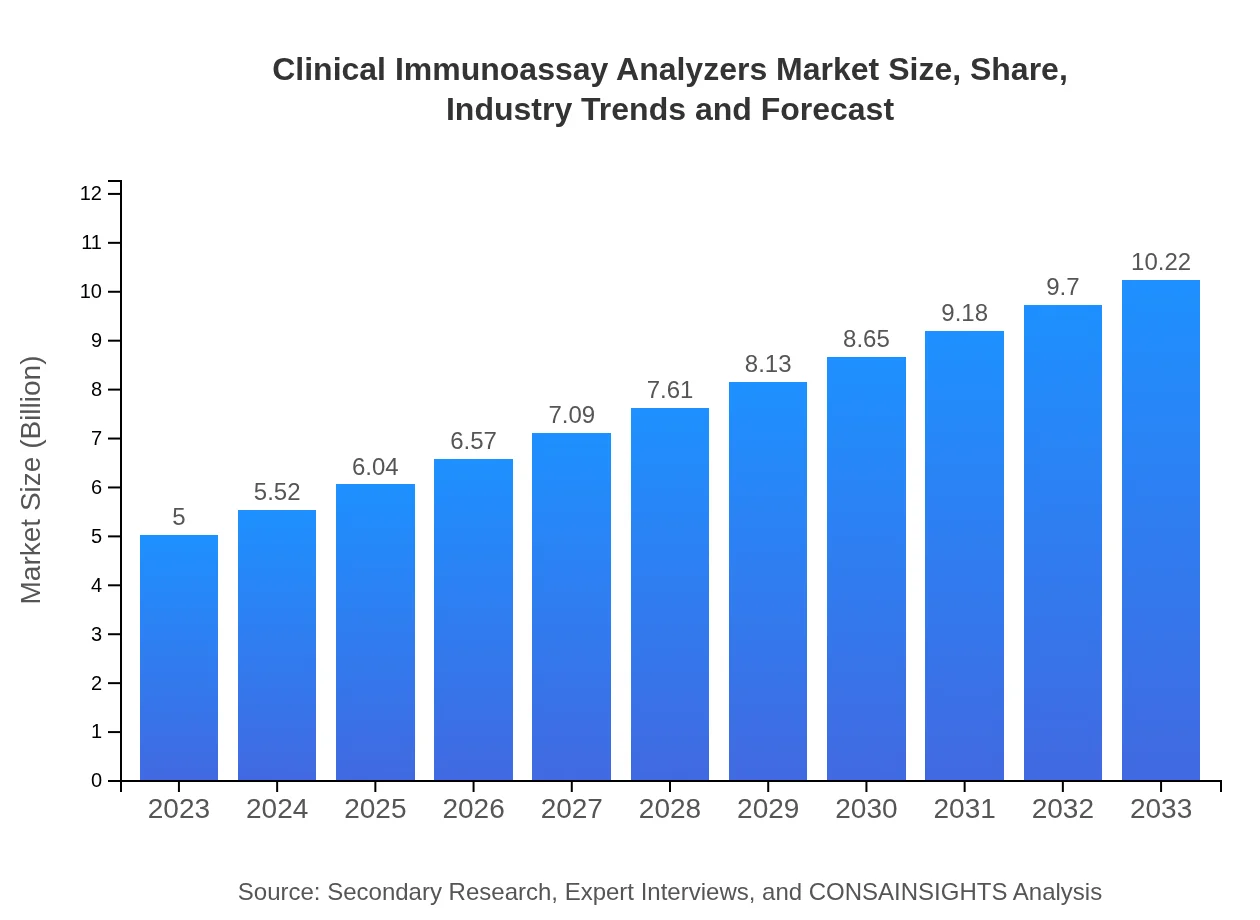
Clinical Immunoassay Analyzers Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Clinical Immunoassay Analyzers market, exploring market size, growth forecasts, industry dynamics, and regional insights from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.00 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, Bio-Rad Laboratories |
| Last Modified Date | 31 January 2026 |
Clinical Immunoassay Analyzers Market Overview
Customize Clinical Immunoassay Analyzers Market Report market research report
- ✔ Get in-depth analysis of Clinical Immunoassay Analyzers market size, growth, and forecasts.
- ✔ Understand Clinical Immunoassay Analyzers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clinical Immunoassay Analyzers
What is the Market Size & CAGR of Clinical Immunoassay Analyzers market in 2023?
Clinical Immunoassay Analyzers Industry Analysis
Clinical Immunoassay Analyzers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clinical Immunoassay Analyzers Market Analysis Report by Region
Europe Clinical Immunoassay Analyzers Market Report:
In Europe, the market size is expected to grow significantly from $1.64 billion in 2023 to $3.35 billion by 2033, fueled by increasing investments in advanced diagnostic technologies and a growing elderly population.Asia Pacific Clinical Immunoassay Analyzers Market Report:
The Asia Pacific region is projected to witness robust growth, with the market size anticipated to grow from $0.82 billion in 2023 to $1.67 billion by 2033. The increasing focus on improving healthcare infrastructure and rising government initiatives in diagnostics play a crucial role in driving this growth.North America Clinical Immunoassay Analyzers Market Report:
Dominating the market, North America is projected to increase from $1.83 billion in 2023 to $3.73 billion by 2033. The presence of major market players and advancements in healthcare technologies are key growth drivers.South America Clinical Immunoassay Analyzers Market Report:
The South American market, though smaller, is expected to double, rising from $0.14 billion in 2023 to $0.28 billion in 2033. Factors such as improving economic conditions and rising healthcare investments are contributing to the growth.Middle East & Africa Clinical Immunoassay Analyzers Market Report:
The Middle East and Africa region is projected to grow from $0.58 billion in 2023 to $1.19 billion by 2033, driven by rising demand for sophisticated laboratory equipment and the expansion of healthcare facilities.Tell us your focus area and get a customized research report.
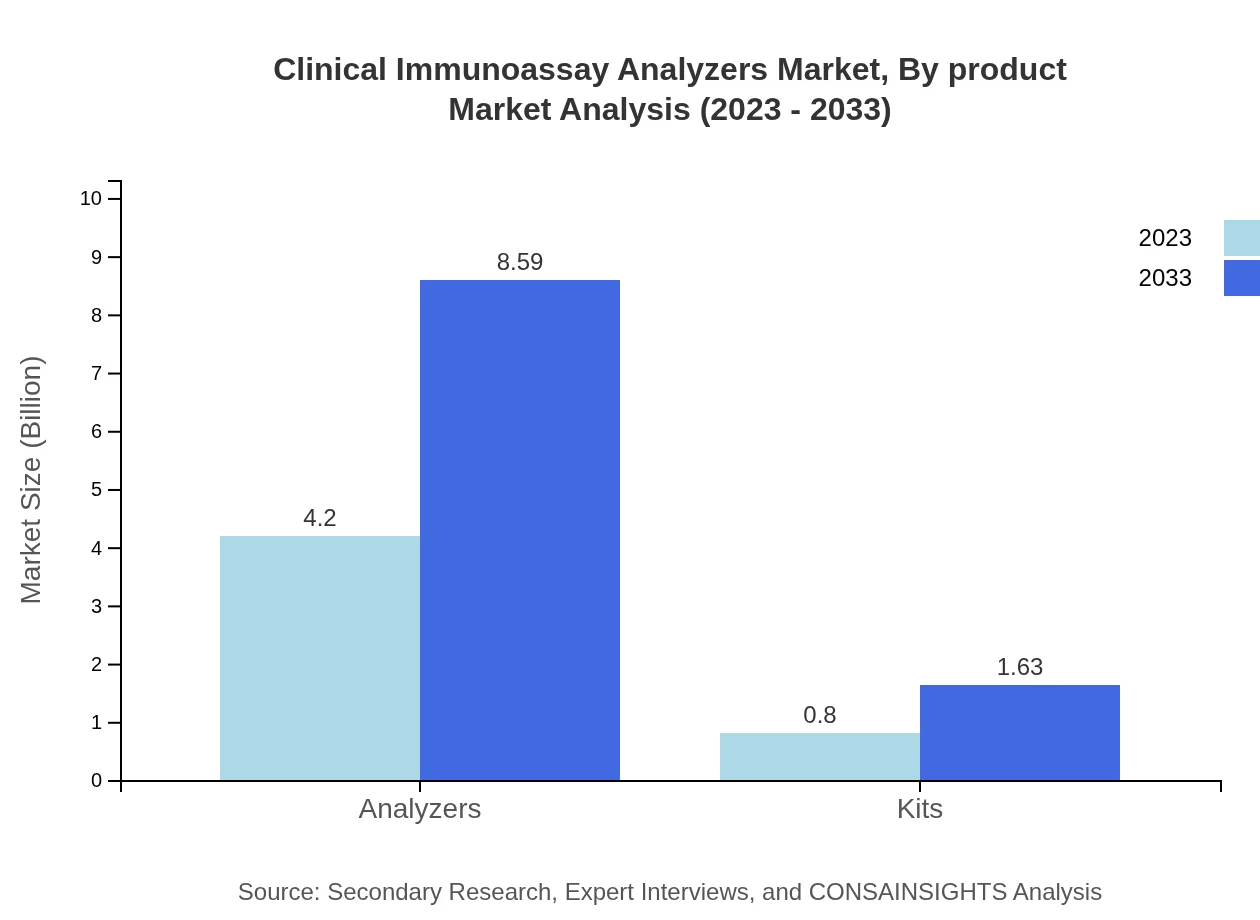
Clinical Immunoassay Analyzers Market Analysis By Product
The product segment dominates the market, particularly analyzers, which accounted for $4.20 billion in 2023 and are expected to reach $8.59 billion by 2033, maintaining an 84.01% market share.
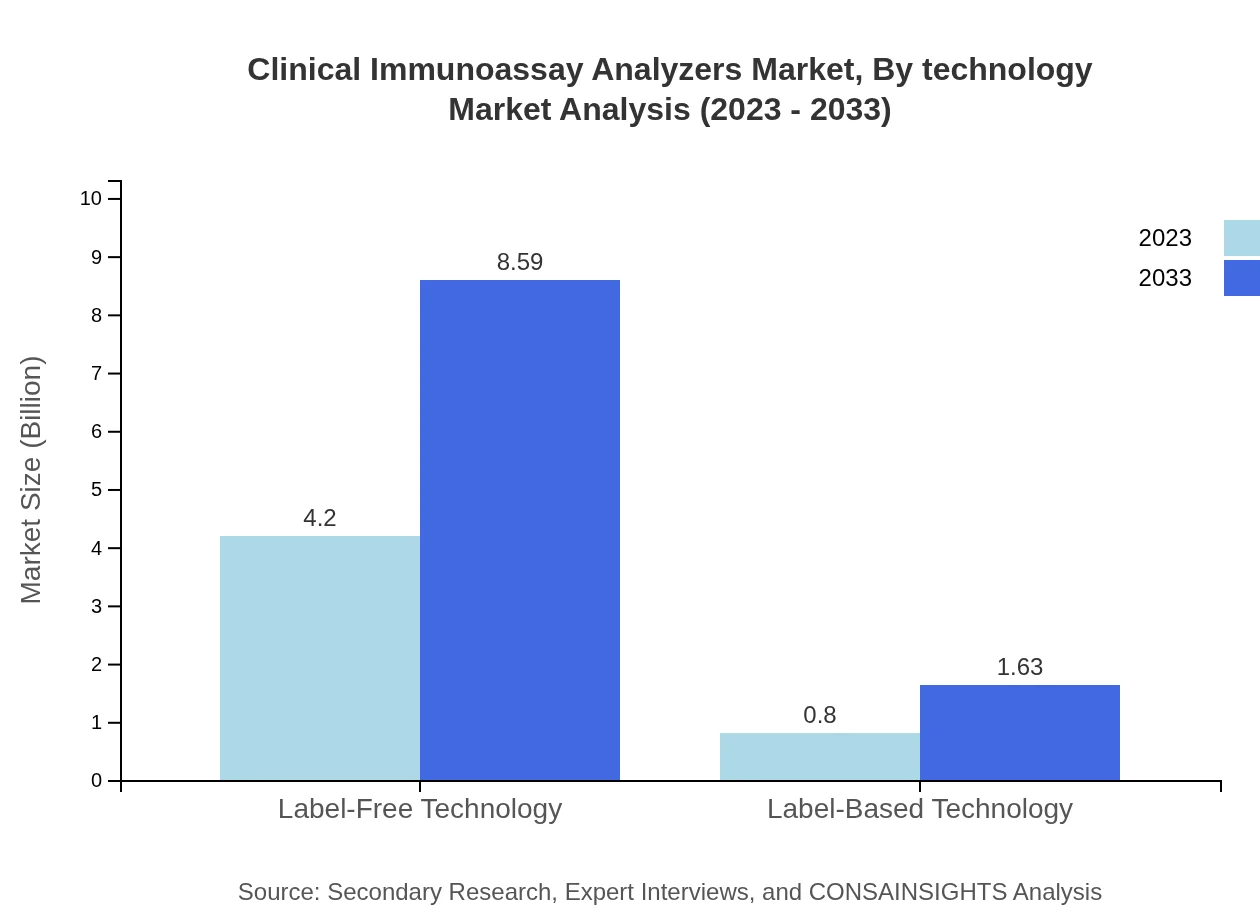
Clinical Immunoassay Analyzers Market Analysis By Technology
Label-free technology accounted for $4.20 billion in 2023, with a projected increase to $8.59 billion by 2033. Label-based technology is also witnessing growth, with a market size expected to rise from $0.80 billion to $1.63 billion over the same period.
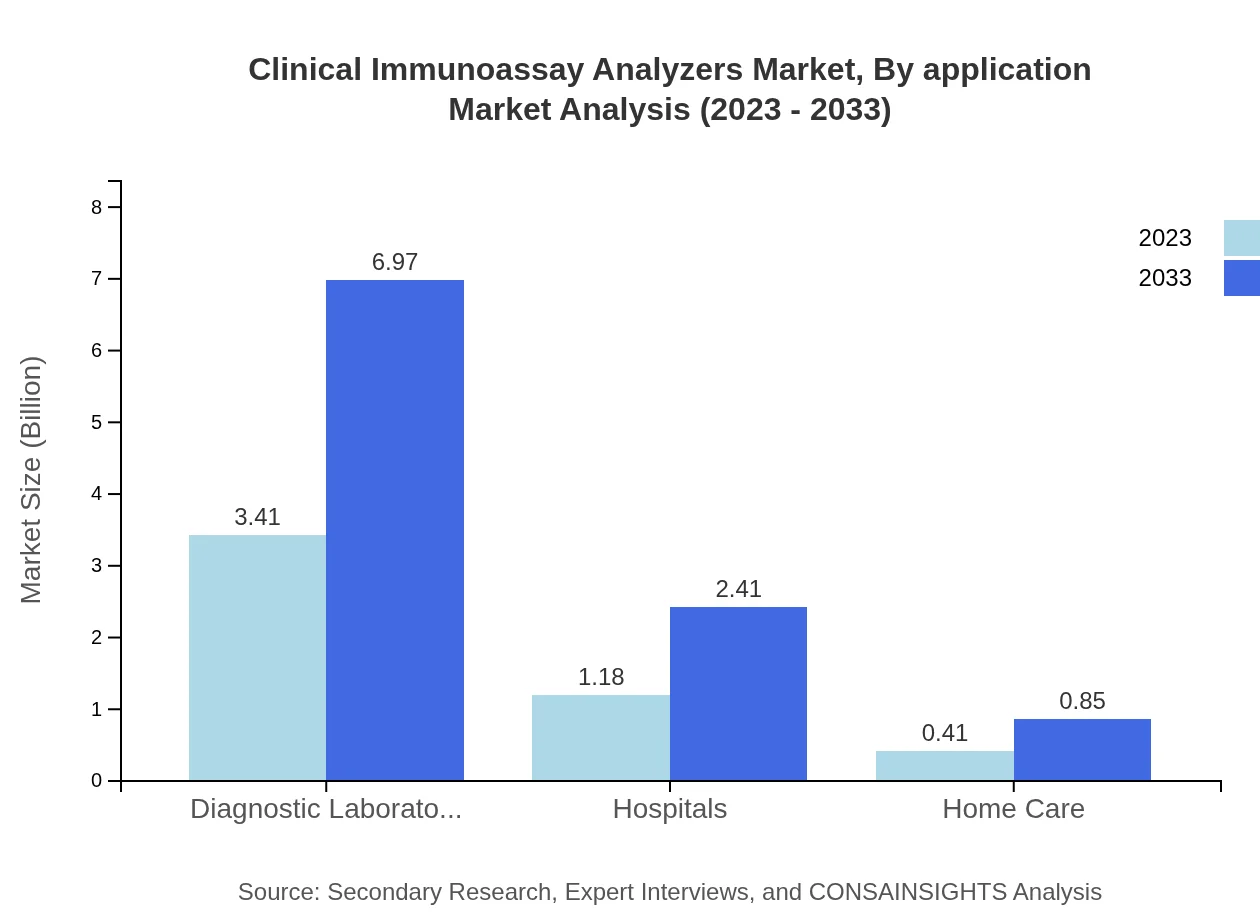
Clinical Immunoassay Analyzers Market Analysis By Application
Applications in clinical diagnostics are significant, particularly in clinical laboratories which held a market size of $3.41 billion in 2023, growing to $6.97 billion by 2033. The pharmaceutical companies segment also reflects notable growth.
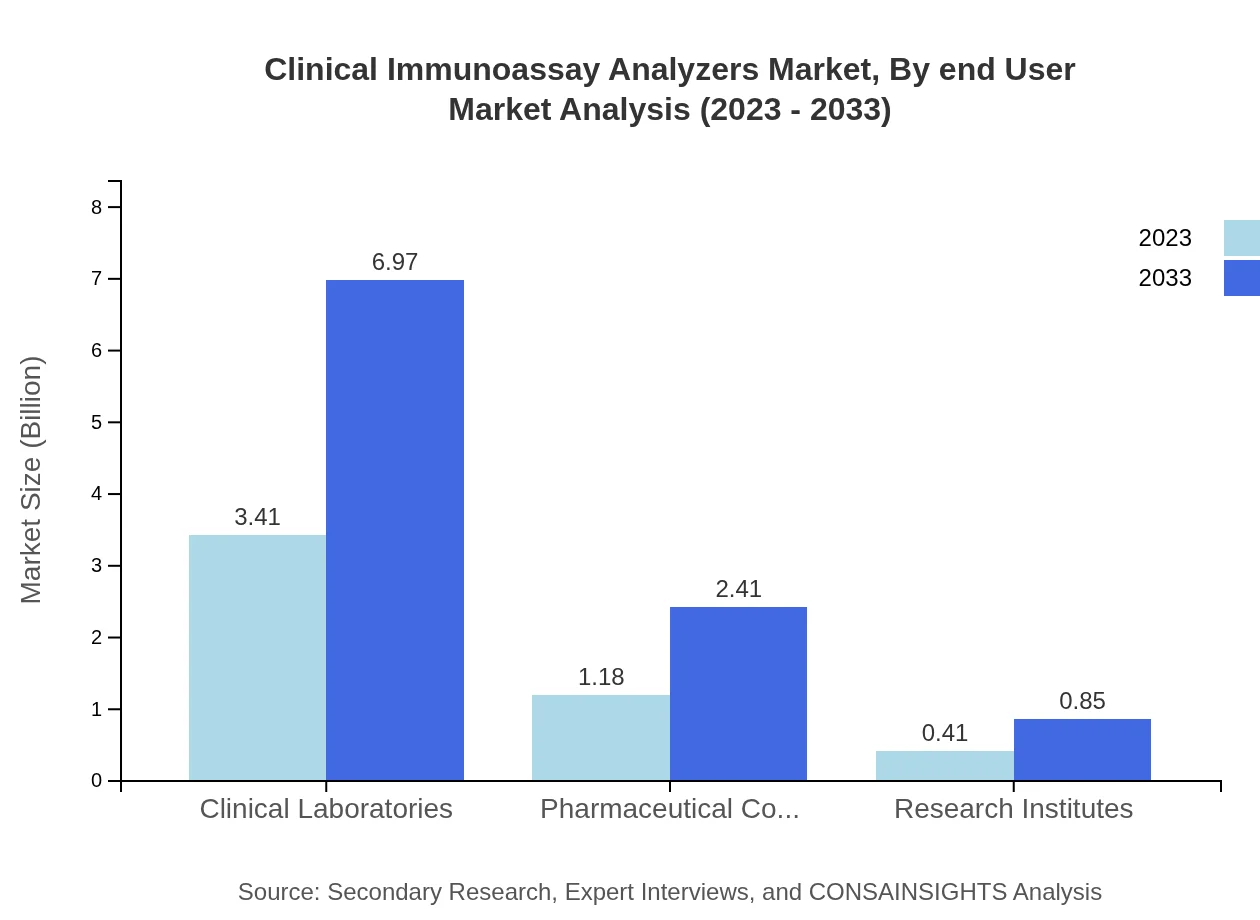
Clinical Immunoassay Analyzers Market Analysis By End User
Hospitals are primary users of clinical immunoassay analyzers, expected to grow from $1.18 billion in 2023 to $2.41 billion by 2033, contributing 23.56% of the market share.
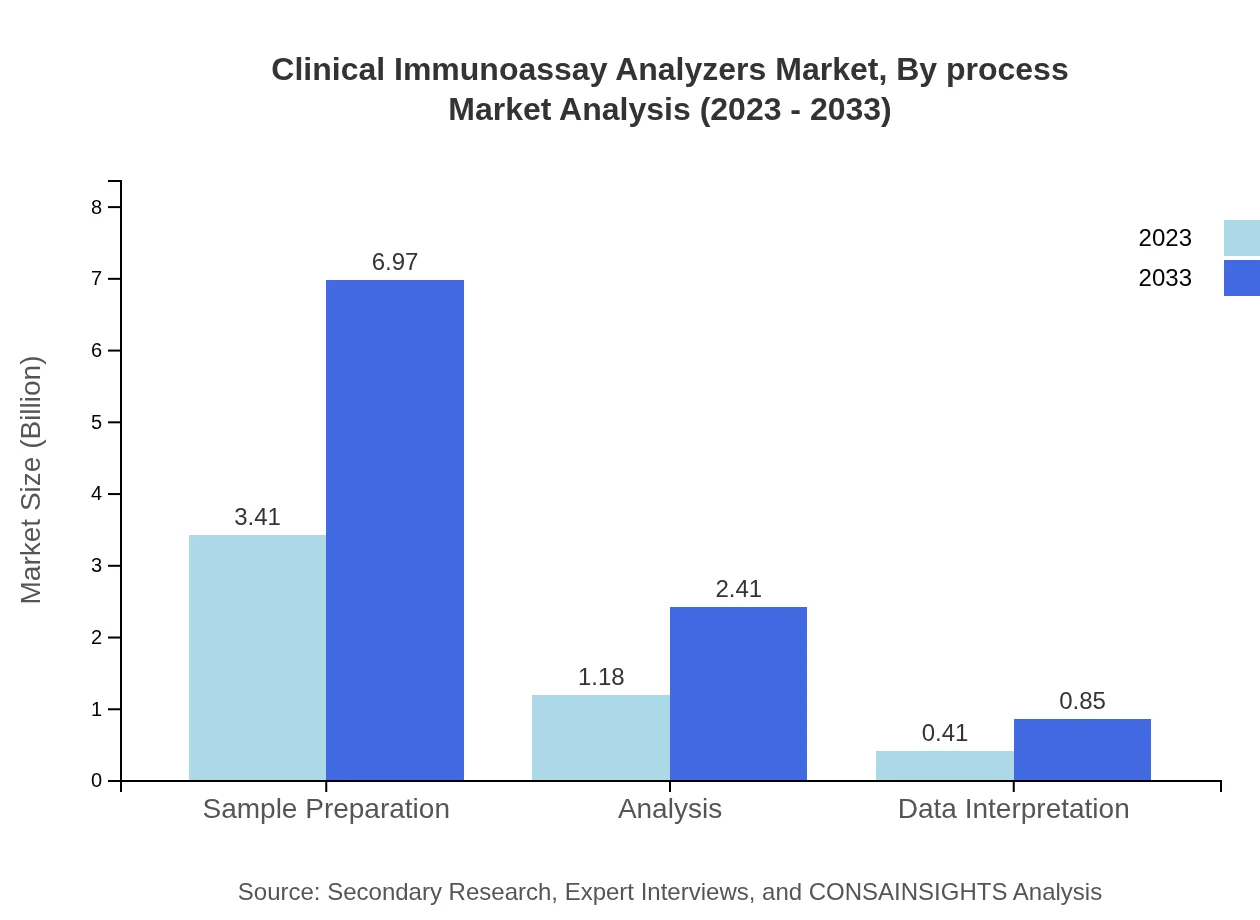
Clinical Immunoassay Analyzers Market Analysis By Process
Sample preparation processes are crucial, which are projected to grow from $3.41 billion in 2023 to $6.97 billion by 2033, highlighting the significance in operational efficiency in diagnostics.
Clinical Immunoassay Analyzers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clinical Immunoassay Analyzers Industry
Abbott Laboratories:
Abbott is a global healthcare company known for its advanced diagnostic solutions, particularly in immunoassays, with a strong emphasis on innovation in laboratory technologies.Roche Diagnostics:
Roche Diagnostics is a leader in in vitro diagnostics, offering a wide range of immunoassay analyzers and related products known for their reliability and accuracy.Siemens Healthineers:
Siemens Healthineers provides a robust portfolio of diagnostic imaging and laboratory diagnostics, including innovative immunoassay analyzers that enhance diagnostic testing efficiency.Thermo Fisher Scientific:
Thermo Fisher specializes in serving the healthcare sector with high-quality immunoassay systems that support a variety of diagnostic needs.Bio-Rad Laboratories:
Bio-Rad is focused on providing innovative solutions in life science research and clinical diagnostics, particularly in immunology and hormone assays.We're grateful to work with incredible clients.









FAQs
What is the market size of clinical Immunoassay Analyzers?
The global Clinical Immunoassay Analyzers market is projected to grow from $5 billion in 2023, with a compound annual growth rate (CAGR) of 7.2%, indicating significant expansion opportunities through 2033.
What are the key market players or companies in this clinical Immunoassay Analyzers industry?
Leading players in the Clinical Immunoassay Analyzers market include major companies specializing in diagnostic equipment and technology, contributing to innovations and advancements in immunoassay solutions.
What are the primary factors driving the growth in the clinical Immunoassay Analyzers industry?
Growth in the Clinical Immunoassay Analyzers market is primarily driven by advancements in laboratory diagnostics, increasing prevalence of chronic diseases, and rising demand for accurate and efficient testing methods.
Which region is the fastest Growing in the clinical Immunoassay Analyzers?
The fastest-growing region in the Clinical Immunoassay Analyzers market is Europe, expected to expand from $1.64 billion in 2023 to $3.35 billion by 2033, reflecting robust market dynamics.
Does ConsaInsights provide customized market report data for the clinical Immunoassay Analyzers industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs and research requirements in the clinical-immunoassay-analyzers industry, ensuring relevant insights for decision-making.
What deliverables can I expect from this clinical Immunoassay Analyzers market research project?
Expect comprehensive market analysis, segmented data, competitive landscape insights, and growth forecasts for the Clinical Immunoassay Analyzers market, all structured to facilitate strategic planning.
What are the market trends of clinical Immunoassay Analyzers?
Key trends in the Clinical Immunoassay Analyzers market include the shift towards automation, integration of advanced technologies, and a growing emphasis on personalized medicine in diagnostics.