Clinical Trial Management Systems Market Report
Published Date: 31 January 2026 | Report Code: clinical-trial-management-systems
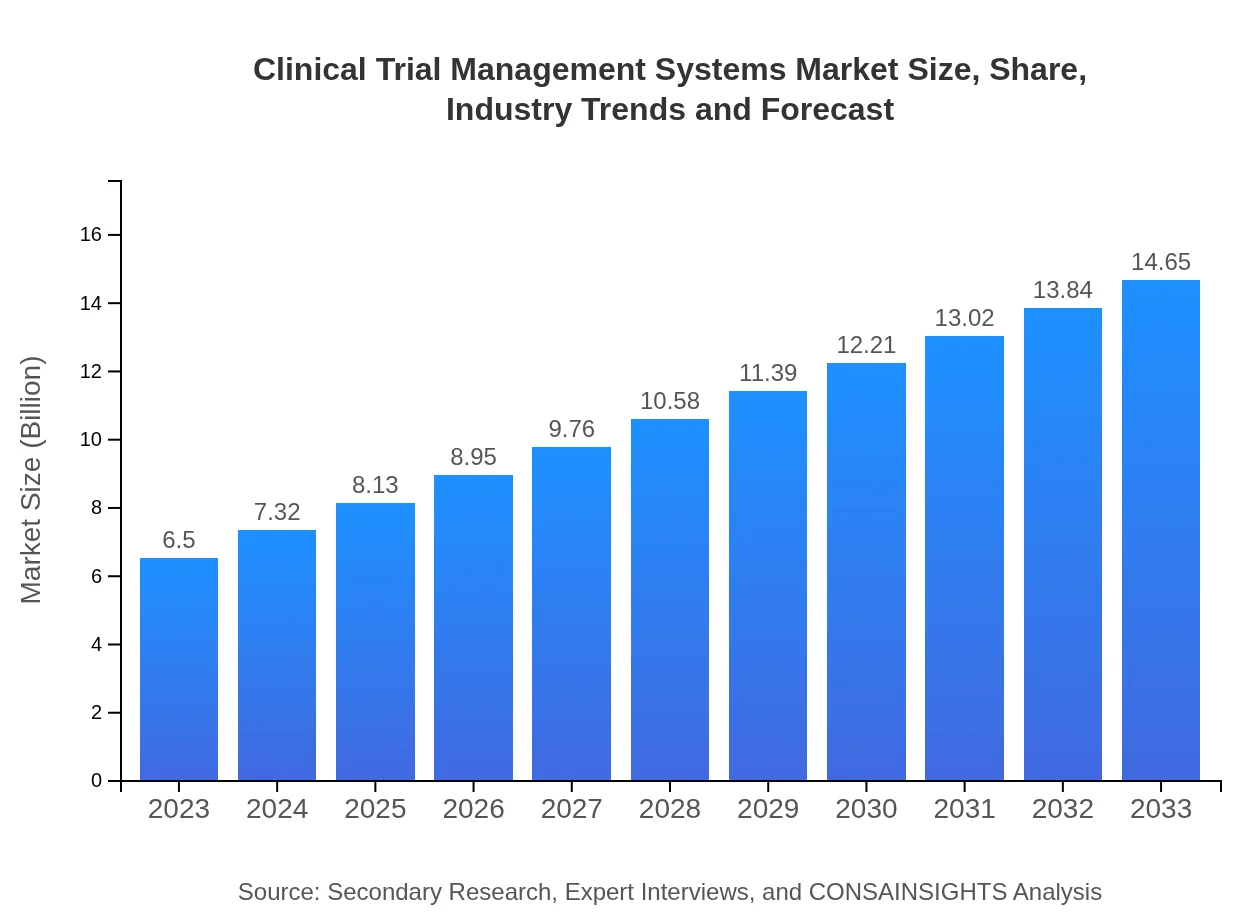
Clinical Trial Management Systems Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Clinical Trial Management Systems market, highlighting trends, insights, and forecasts from 2023 to 2033, covering various segments and regional dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $6.50 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $14.65 Billion |
| Top Companies | Medidata Solutions, Oracle Corporation, Veeva Systems, Medidata, Parexel International |
| Last Modified Date | 31 January 2026 |
Clinical Trial Management Systems Market Overview
Customize Clinical Trial Management Systems Market Report market research report
- ✔ Get in-depth analysis of Clinical Trial Management Systems market size, growth, and forecasts.
- ✔ Understand Clinical Trial Management Systems's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clinical Trial Management Systems
What is the Market Size & CAGR of Clinical Trial Management Systems market in 2023?
Clinical Trial Management Systems Industry Analysis
Clinical Trial Management Systems Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clinical Trial Management Systems Market Analysis Report by Region
Europe Clinical Trial Management Systems Market Report:
In Europe, the CTMS market is expected to grow from $2.01 billion in 2023 to approximately $4.52 billion by 2033. A significant factor behind this growth is the rising focus on drug development and innovative therapies, along with stringent regulatory compliance in clinical trials.Asia Pacific Clinical Trial Management Systems Market Report:
The Asia-Pacific region's CTMS market is valued at $1.27 billion in 2023, estimated to grow to $2.86 billion by 2033. The rise in clinical trial activity and supportive government initiatives encourage market growth, while large pharmaceuticals are increasingly setting up R&D facilities in this region.North America Clinical Trial Management Systems Market Report:
The North American market, valued at $2.17 billion in 2023, is forecasted to reach $4.90 billion by 2033. The region hosts key market players and benefits from advanced technological infrastructure, regulatory framework, and an increasing number of clinical trials conducted.South America Clinical Trial Management Systems Market Report:
With a valuation of $0.43 billion in 2023, the South American CTMS market is projected to reach $0.97 billion by 2033. Economic improvements, coupled with an increasing focus on healthcare infrastructure and regulatory reforms, are expected to drive market growth.Middle East & Africa Clinical Trial Management Systems Market Report:
The Middle East and Africa market is expected to grow from $0.62 billion in 2023 to $1.40 billion by 2033. This growth is driven by increasing investments in healthcare infrastructure and a growing number of clinical studies being initiated across the region.Tell us your focus area and get a customized research report.
Clinical Trial Management Systems Market Analysis By Solution
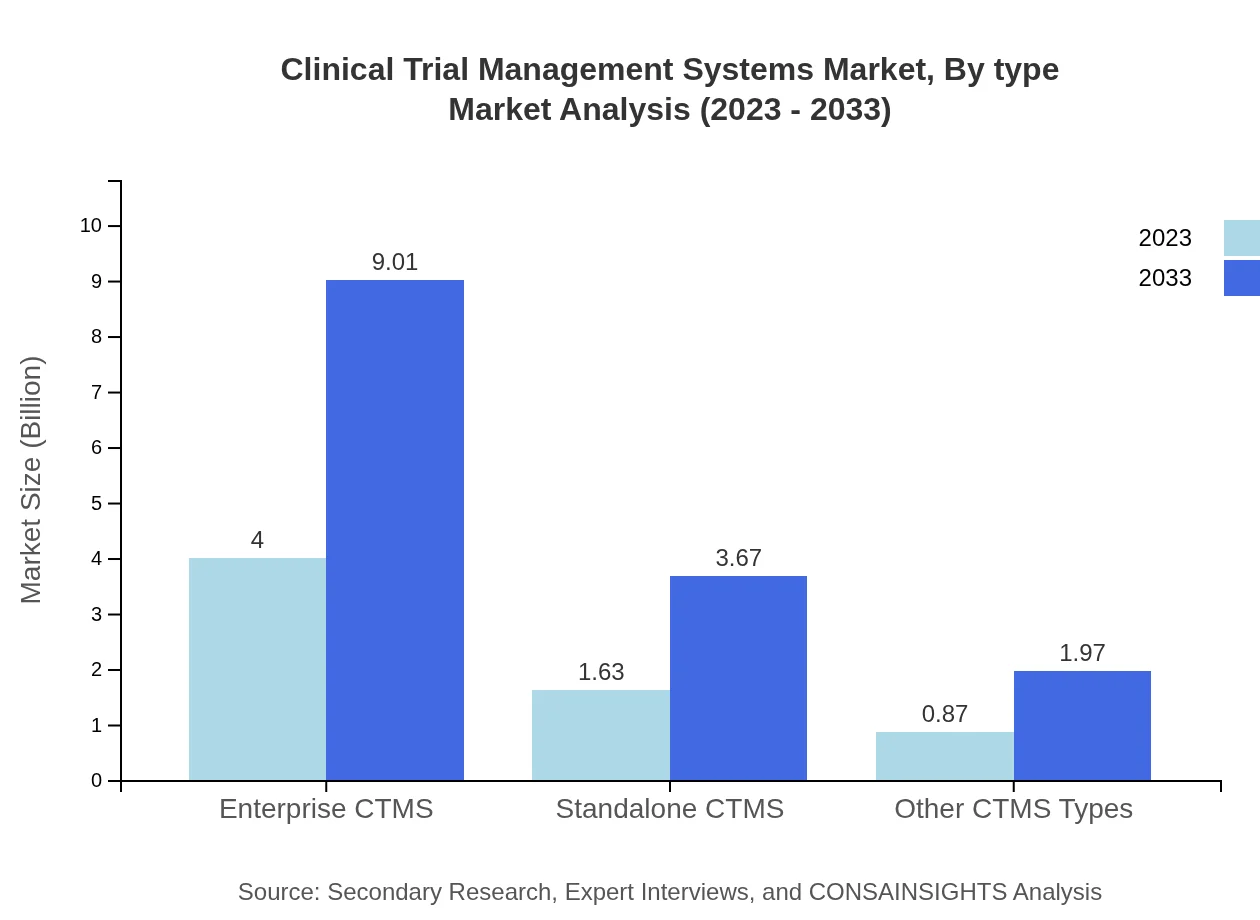
The market is segmented into enterprise CTMS, standalone CTMS, and other CTMS types. By 2033, enterprise CTMS is expected to dominate with a market share of 61.5%, reflecting established adoption in large pharmaceutical companies. Standalone CTMS is projected to reach $3.67 billion by 2033, capturing a significant share for specific project needs. Other types of CTMS also gain traction, with projected revenue of $1.97 billion.
Clinical Trial Management Systems Market Analysis By Type
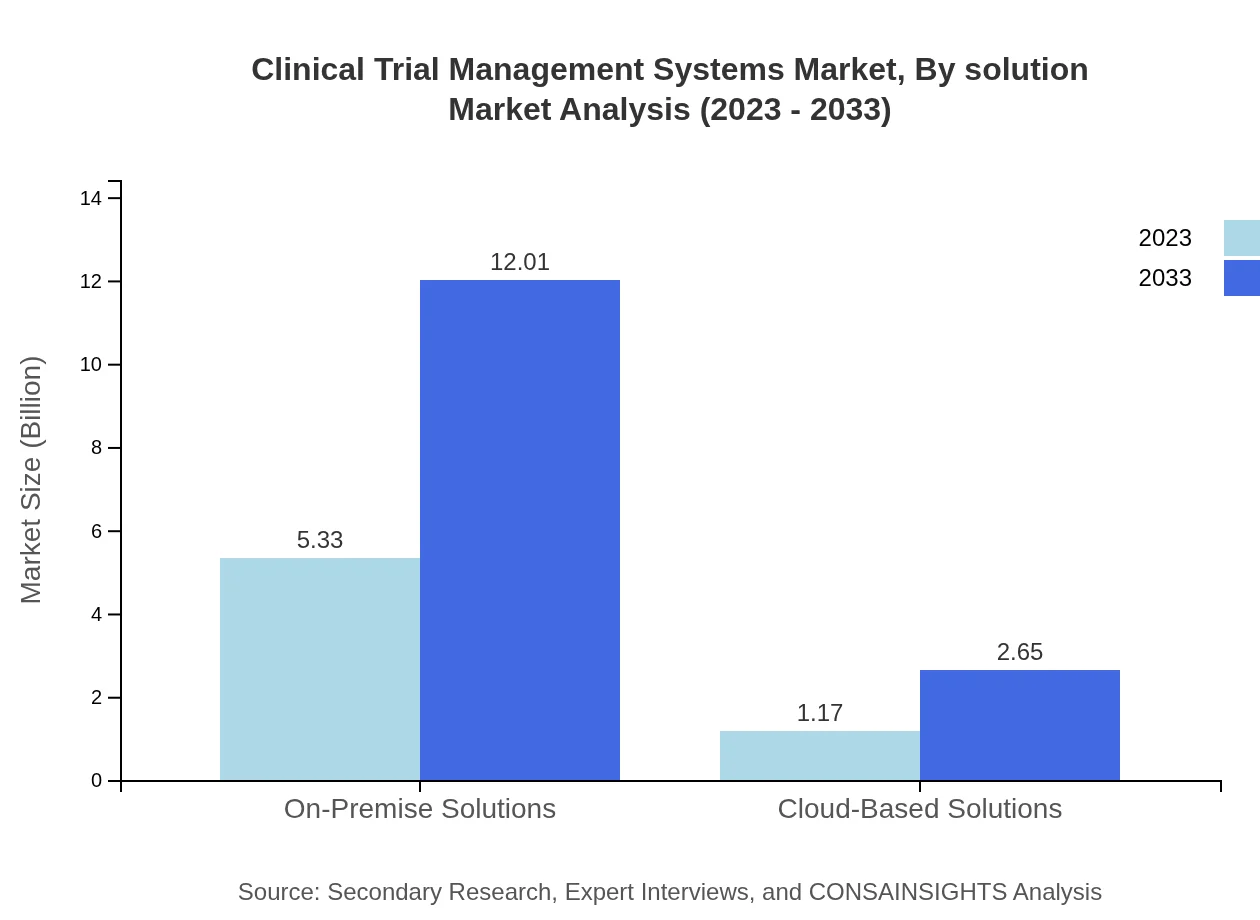
The Clinical Trial Management Systems market is segmented into on-premise solutions and cloud-based solutions. The on-premise deployment is projected to continue leading with a market value estimated to reach $12.01 billion by 2033, driven by organizations preferring in-house data management for compliance reasons. In contrast, cloud-based solutions are anticipated to grow, reaching a value of $2.65 billion as organizations migrate to more flexible, scalable options.
Clinical Trial Management Systems Market Analysis By End User
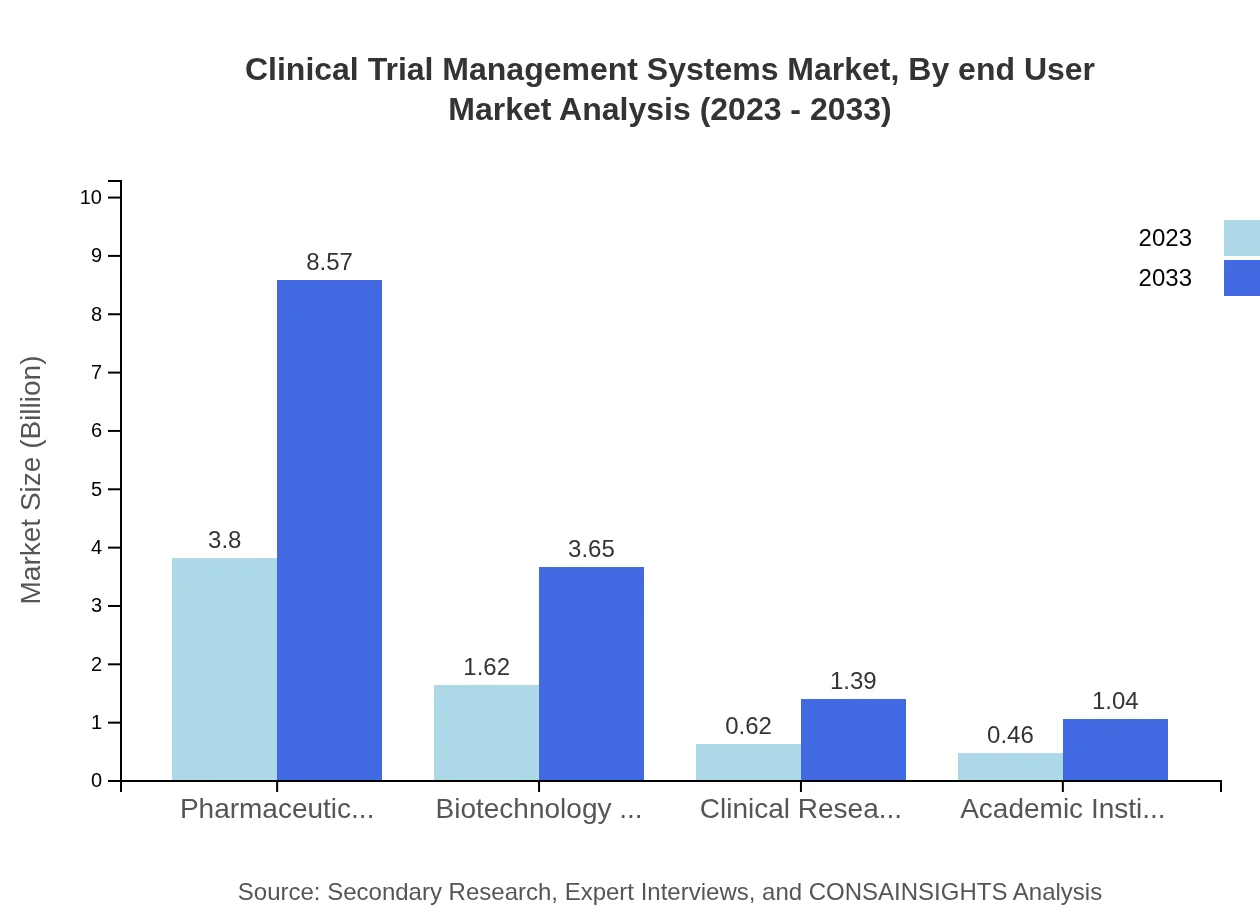
In terms of end-users, pharmaceutical companies hold the dominant market share of 58.48% and are expected to reach $8.57 billion by 2033, due to high R&D investments. Biotechnology firms also represent a significant market segment, with growth anticipated to reach 3.65 billion. Clinical Research Organizations are expected to expand their share in using CTMS solutions, projected to reach $1.39 billion by the end of the forecast period.
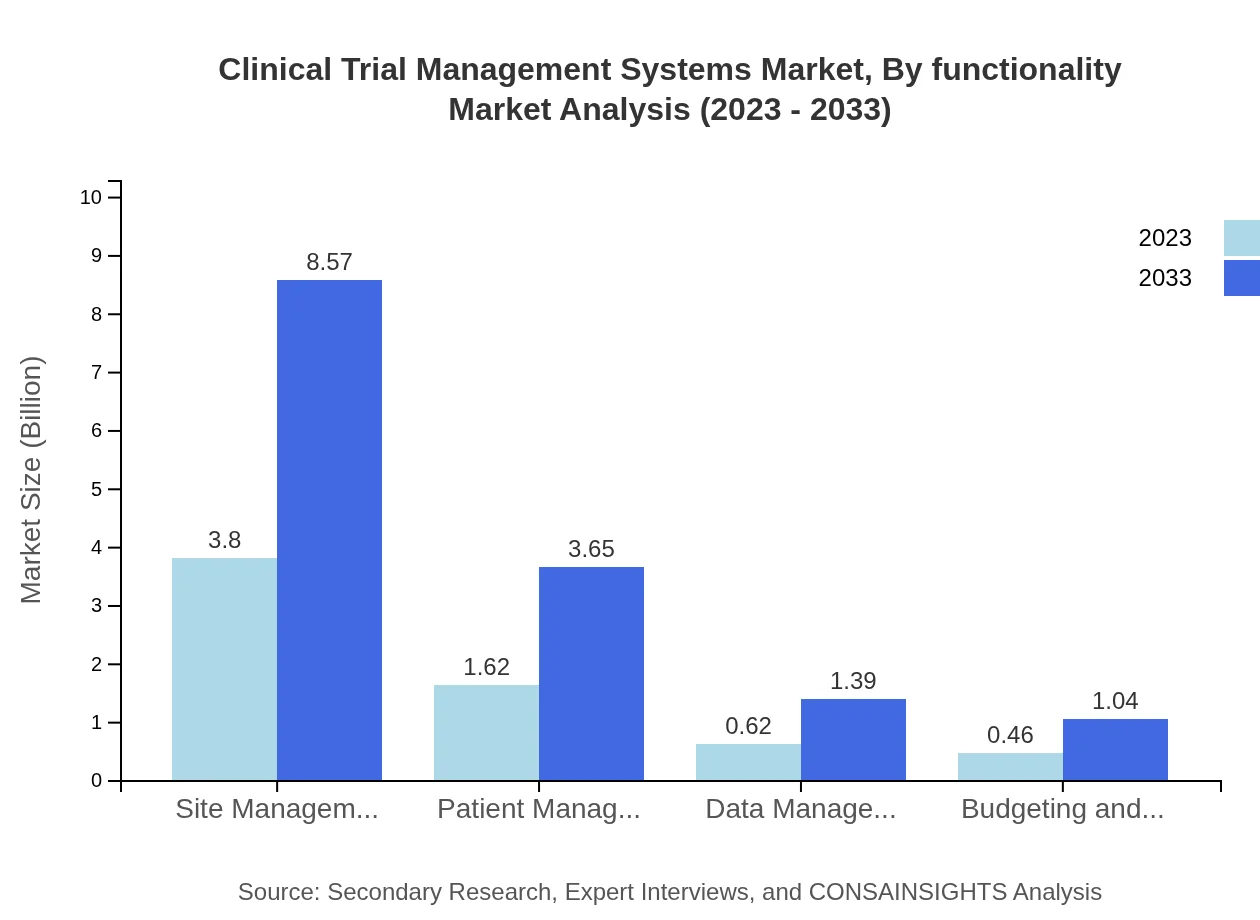
Clinical Trial Management Systems Market Analysis By Functionality
Segmented by functionality, site management holds a significant market share at 58.48%, focusing on managing sites effectively throughout clinical trials. Patient management is also notable, projected to reach $3.65 billion by 2033, as organizations prioritize patient-centric trial designs. Other functionalities include data management, budgeting, and forecasting, addressing all aspects of trial management effectively.
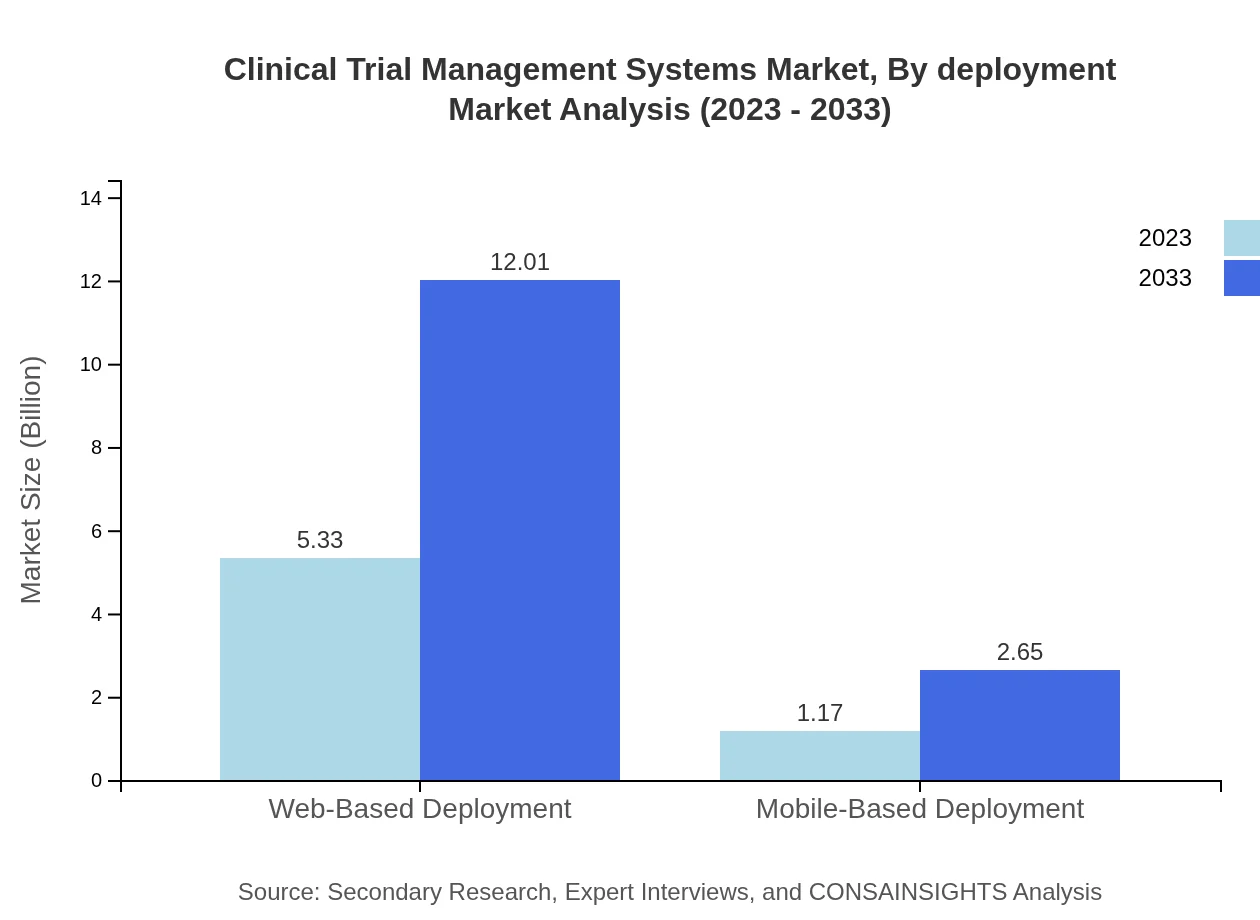
Clinical Trial Management Systems Market Analysis By Deployment
The Clinical Trial Management Systems market favors web-based deployments, expected to capture 81.94% of the market by reaching $12.01 billion by 2033. This preference reflects enhanced user accessibility and integration. Mobile-based solutions, while a smaller segment, are projected to grow to $2.65 billion, catering to an increasingly mobile workforce.
Clinical Trial Management Systems Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clinical Trial Management Systems Industry
Medidata Solutions:
A leader in the life science industry, providing cloud-based solutions for clinical trials and an extensive CTMS platform that enhances operational efficiency.Oracle Corporation:
Offering comprehensive and integrated CTMS solutions, Oracle is a pivotal player in the biotechnology and pharmaceutical sectors, known for its data management capabilities.Veeva Systems:
Known for cloud-based software solutions, Veeva specializes in applications designed specifically for the life sciences industry, including CTMS that streamline trial processes.Medidata:
Medidata provides a global cloud platform designed for clinical trials, focusing on data analytics and collaboration to enhance trial outcomes.Parexel International:
A worldwide leader in contract research, Parexel's CTMS offerings focus on helping organizations optimize trial performance and streamline processes.We're grateful to work with incredible clients.









FAQs
What is the market size of clinical Trial Management Systems?
The Clinical Trial Management Systems (CTMS) market is valued at $6.5 billion in 2023, with an impressive compound annual growth rate (CAGR) of 8.2%. This growth indicates a robust demand for efficient clinical trial management solutions.
What are the key market players or companies in the clinical Trial Management Systems industry?
Key players in the CTMS market include major pharmaceutical firms, biotechnology companies, Clinical Research Organizations (CROs), and various IT vendors. These companies heavily influence the market dynamics through innovation and strategic partnerships.
What are the primary factors driving the growth in the clinical Trial Management Systems industry?
Growth drivers for the CTMS industry include the increasing complexity of clinical trials, regulatory pressures, the need for data integrity, and technological advancements. Rising investments in R&D by pharmaceutical companies further accelerate market expansion.
Which region is the fastest Growing in the clinical Trial Management Systems?
The Asia Pacific region is the fastest-growing market for CTMS, expected to expand from $1.27 billion in 2023 to $2.86 billion by 2033. This growth is fueled by increasing clinical trials and investment in healthcare infrastructure.
Does ConsaInsights provide customized market report data for the clinical Trial Management Systems industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the CTMS industry. Clients can expect detailed insights, segmented market analysis, and thorough research methodologies to inform strategic decisions.
What deliverables can I expect from this clinical Trial Management Systems market research project?
Deliverables from the CTMS market research project include comprehensive reports, market sizing data, segmentation analysis, competitive landscape, and insights on emerging trends. Clients receive actionable recommendations to help inform their strategic planning.
What are the market trends of clinical Trial Management Systems?
Key trends in the CTMS market include the rise of cloud-based solutions, increased adoption of mobile technologies, and data-driven decision-making. The focus on patient-centric trials and regulatory compliance also shapes future market developments.