Clinical Trials Support Services Market Report
Published Date: 31 January 2026 | Report Code: clinical-trials-support-services
Clinical Trials Support Services Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Clinical Trials Support Services market from 2023 to 2033, encompassing market dynamics, segmentation, regional performance, industry leaders, and future trends.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
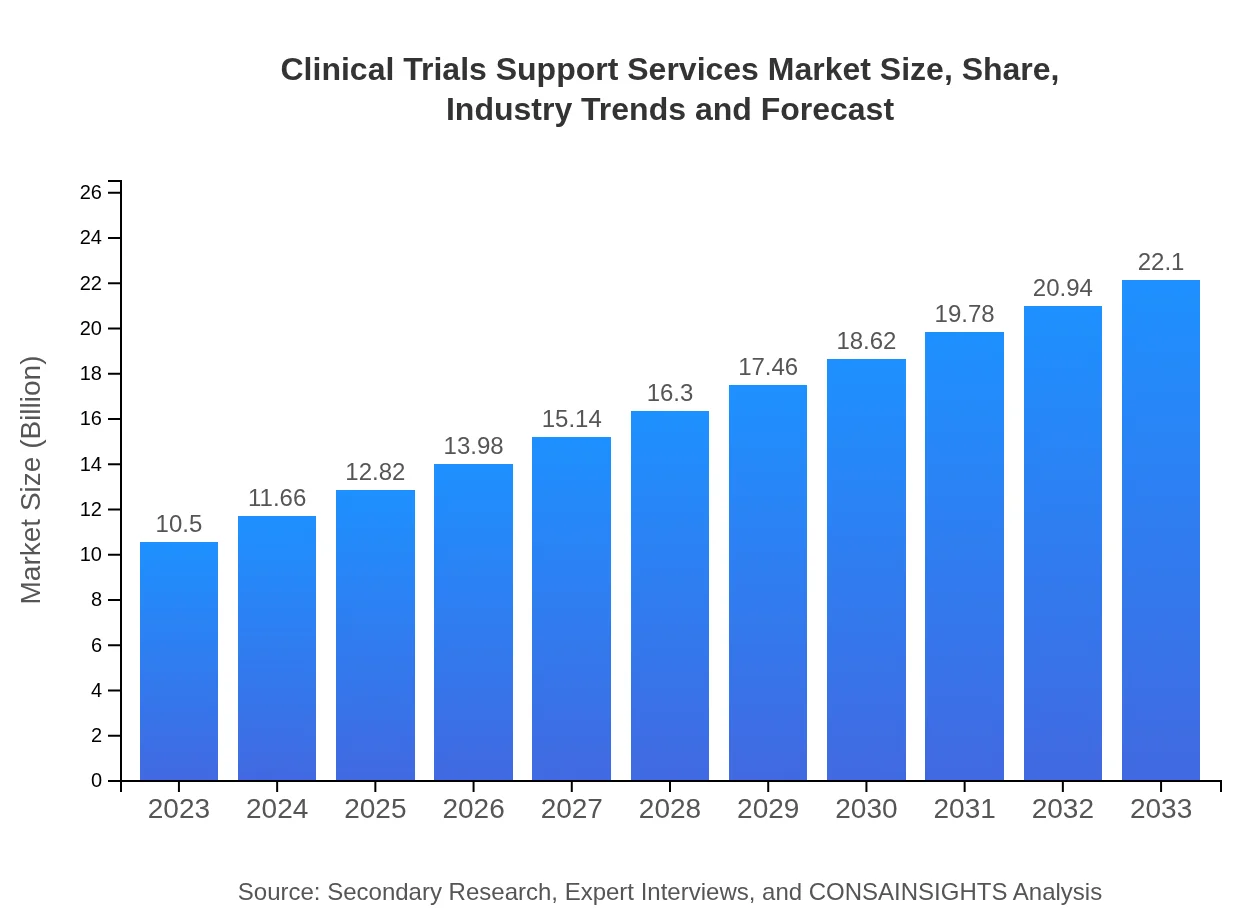
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $22.10 Billion |
| Top Companies | Covance (Labcorp Drug Development), IQVIA , PAREXEL International, Charles River Laboratories |
| Last Modified Date | 31 January 2026 |
Clinical Trials Support Services Market Overview
Customize Clinical Trials Support Services Market Report market research report
- ✔ Get in-depth analysis of Clinical Trials Support Services market size, growth, and forecasts.
- ✔ Understand Clinical Trials Support Services's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clinical Trials Support Services
What is the Market Size & CAGR of Clinical Trials Support Services market in 2023?
Clinical Trials Support Services Industry Analysis
Clinical Trials Support Services Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clinical Trials Support Services Market Analysis Report by Region
Europe Clinical Trials Support Services Market Report:
In Europe, the market is valued at approximately $3.13 billion in 2023, with expectations to grow to $6.59 billion by 2033. The region benefits from established clinical research networks and comprehensive regulatory frameworks. The presence of skilled workforce and increasing partnerships among pharmaceutical companies, contract research organizations (CROs), and academic institutions enhance the overall capabilities of the market in Europe.Asia Pacific Clinical Trials Support Services Market Report:
The Asia Pacific region displays immense potential for growth within the Clinical Trials Support Services market, primarily driven by increasing investments from global pharmaceutical companies and a growing number of clinical trials conducted in countries like China and India. In 2023, the market is valued at $2.08 billion and is anticipated to grow to $4.38 billion by 2033. The region's diverse population provides a substantial patient pool, facilitating faster trial recruitment and improved timelines.North America Clinical Trials Support Services Market Report:
North America remains the dominant player in the Clinical Trials Support Services market, with a market size of $3.67 billion in 2023 projected to grow to $7.72 billion by 2033. The region is characterized by a high concentration of pharmaceutical companies, robust funding for drug development, and advanced clinical research infrastructure. The increasing trend of outsourcing clinical trials to specialized service providers is also a key driver of growth.South America Clinical Trials Support Services Market Report:
South America is gradually emerging as a favorable landscape for clinical trials, thanks to supportive regulatory frameworks and collaboration between local institutions and international sponsors. The market in this region was valued at $0.89 billion in 2023 and is expected to reach $1.88 billion by 2033. Countries such as Brazil and Argentina are leading the charge, offering opportunities for innovative trials in infectious diseases and chronic conditions.Middle East & Africa Clinical Trials Support Services Market Report:
The Clinical Trials Support Services market in the Middle East and Africa is at a nascent stage but is poised for growth, given increasing health needs, rising investments in clinical infrastructure, and a push towards research-oriented healthcare systems. In 2023, the market is valued at $0.73 billion, with projections to reach $1.54 billion by 2033. Emerging markets like South Africa and the UAE are expected to see significant advancements in the clinical trials landscape.Tell us your focus area and get a customized research report.
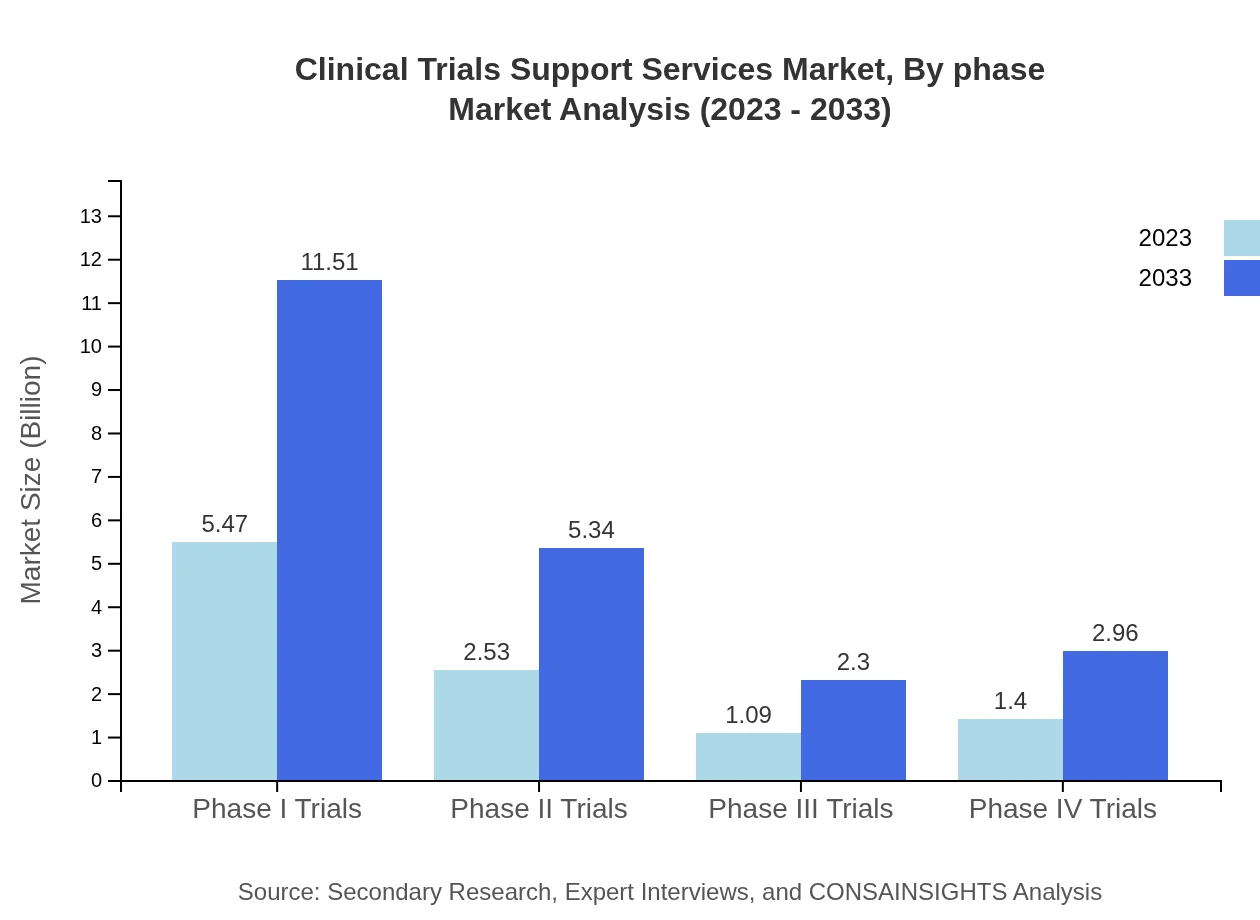
Clinical Trials Support Services Market Analysis By Phase
The market is segmented based on the phases of clinical trials, with Phase I Trials valued at $5.47 billion in 2023 and projected to grow to $11.51 billion by 2033, maintaining a 52.07% share of the market. Phase II Trials are valued at $2.53 billion with growth to $5.34 billion, holding a share of 24.14%. Phase III Trials currently valued at $1.09 billion are expected to expand to $2.30 billion, while Phase IV Trials grow from $1.40 billion to $2.96 billion, equally significant in the trial process.
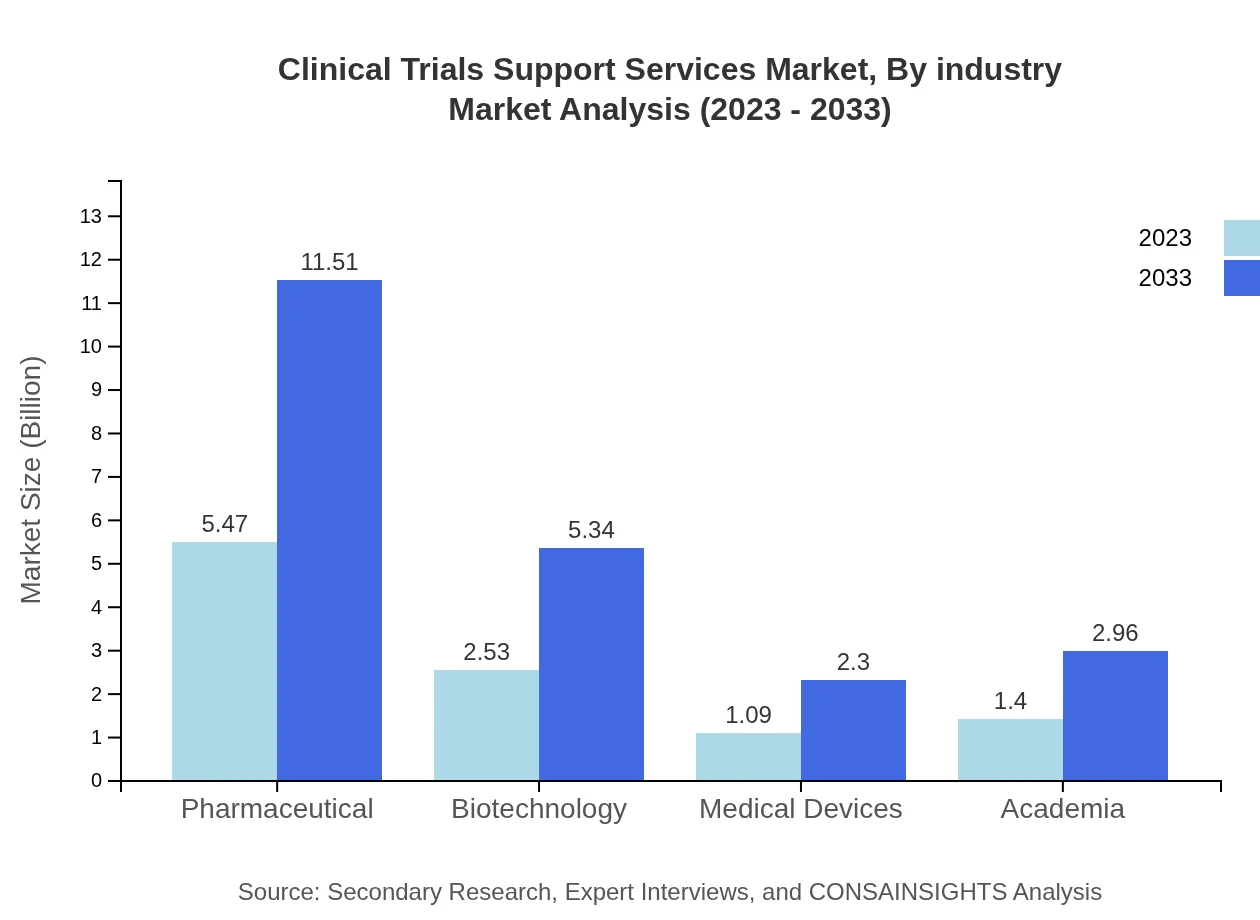
Clinical Trials Support Services Market Analysis By Industry
In terms of industry segmentation, the pharmaceutical sector holds a 52.07% share, valued at $5.47 billion in 2023 and projected to rise to $11.51 billion. The biotechnology sector accounts for 24.14% of revenue, starting at $2.53 billion and expanding to $5.34 billion over the forecast period. Medical devices contribute a 10.42% share, with market figures growing from $1.09 billion to $2.30 billion, while academia holds a 13.37% share moving from $1.40 billion to $2.96 billion.
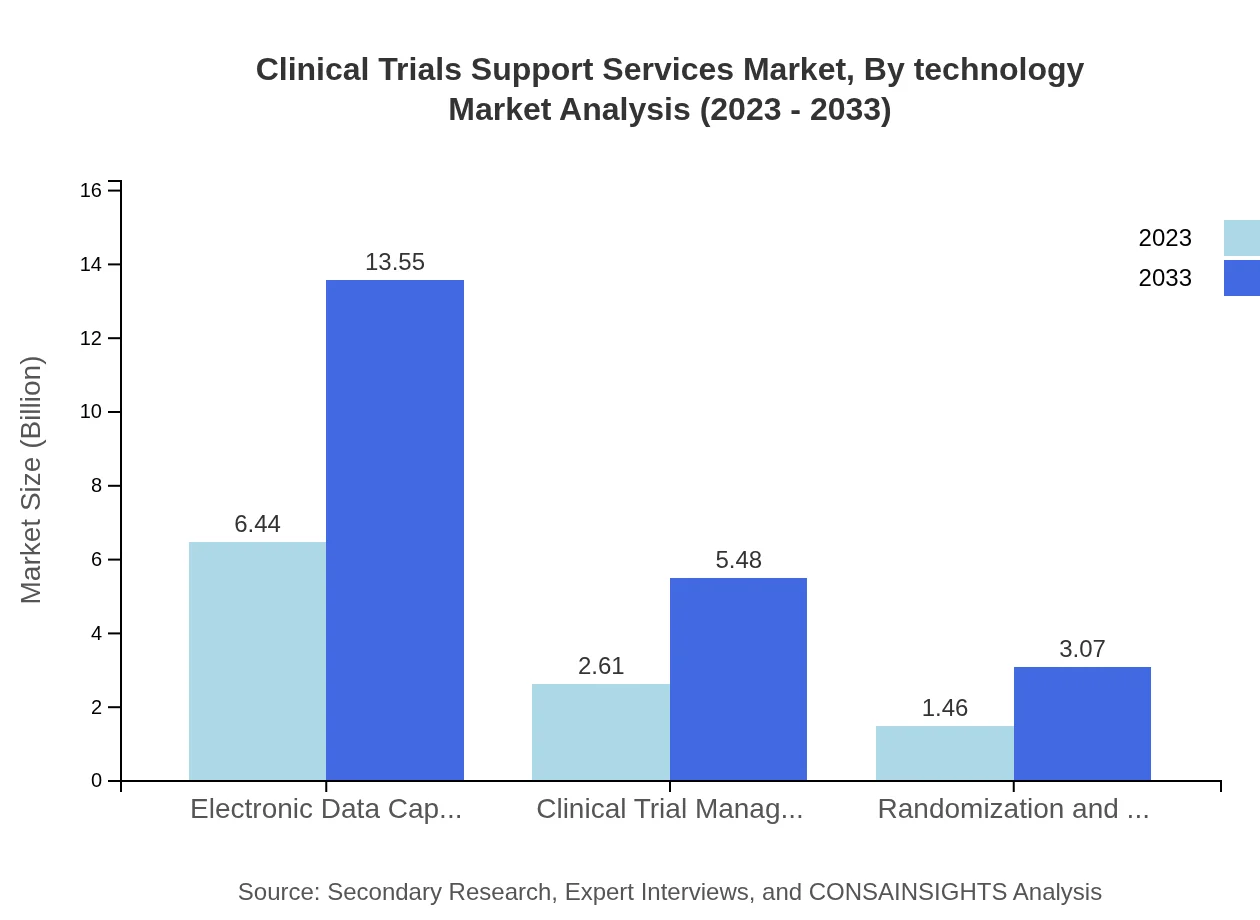
Clinical Trials Support Services Market Analysis By Technology
Emerging technologies are reshaping the Clinical Trials Support Services landscape. Notably, Electronic Data Capture (EDC) holds a leading position, valued at $6.44 billion in 2023 and set to reach $13.55 billion by 2033, making up 61.32% of the market. Clinical Trial Management Systems (CTMS) size is anticipated to grow from $2.61 billion to $5.48 billion, while Randomization and Trial Supply Management (RTSM) services are expected to move from $1.46 billion to $3.07 billion, reflecting substantial reliance on these technologies for efficient trial management.
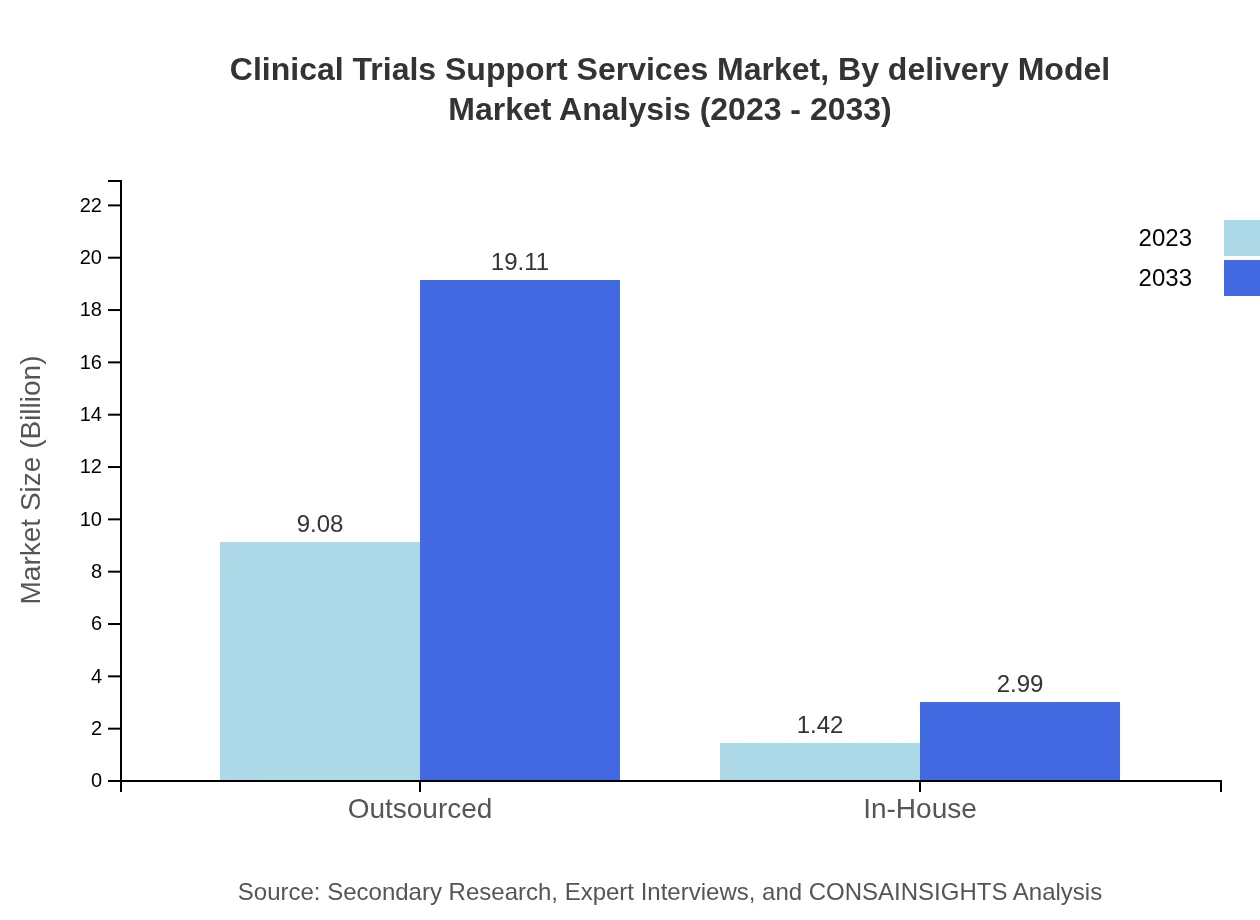
Clinical Trials Support Services Market Analysis By Delivery Model
Delivery models for Clinical Trials Support Services are largely split between outsourced and in-house services. The outsourced segment, valued at $9.08 billion in 2023, is projected to grow to $19.11 billion by 2033, capturing the majority of market share at 86.46%. In-hospital models, however, show potential growth from $1.42 billion to $2.99 billion as organizations seek to maintain some level of in-house capabilities.
Clinical Trials Support Services Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clinical Trials Support Services Industry
Covance (Labcorp Drug Development):
Covance provides comprehensive drug development services, from preclinical studies through Phase IV clinical trials, leveraging data analytics and technology to optimize trial efficiency and outcomes.IQVIA :
IQVIA is a leading global provider of advanced analytics, technology solutions, and contract research services to the life sciences industry, focused on the integration of data and digital platforms.PAREXEL International:
PAREXEL offers extensive expertise in regulatory consulting, clinical research, and post-market surveillance services, helping clients navigate complex conditions in drug development.Charles River Laboratories:
Charles River specializes in a wide array of preclinical and clinical laboratory services for the biopharmaceutical industry, emphasizing innovation and scientific excellence.We're grateful to work with incredible clients.









FAQs
What is the market size of clinical Trials Support Services?
The clinical trials support services market is valued at approximately $10.5 billion in 2023. It is projected to grow at a CAGR of 7.5%, reaching significantly higher figures as the industry expands over the next decade.
What are the key market players or companies in this clinical Trials Support Services industry?
Key players in the clinical trials support services industry include leading pharmaceutical firms, biotech companies, and specialized contract research organizations (CROs) that play a crucial role in facilitating various trial phases and regulatory compliance.
What are the primary factors driving the growth in the clinical trials support Services industry?
Growth in the clinical trials support services industry is driven by an increase in clinical research activities, a rise in chronic diseases, advancements in technology, and heightened focus on patient-centric approaches in drug development.
Which region is the fastest Growing in the clinical Trials Support Services?
The fastest-growing region in the clinical trials support services market is North America. The market in this region reached $3.67 billion in 2023 and is expected to grow to $7.72 billion by 2033, indicating strong demand.
Does ConsaInsights provide customized market report data for the clinical Trials Support Services industry?
Yes, ConsaInsights offers customized market research reports tailored specifically for the clinical trials support services industry, addressing unique client needs and delivering insights that support strategic decision-making processes.
What deliverables can I expect from this clinical Trials Support Services market research project?
Clients can expect comprehensive deliverables including in-depth market analysis, growth forecasts, competitor landscapes, and segment data that guide strategy and enhance understanding of market dynamics.
What are the market trends of clinical Trials Support Services?
Current market trends in clinical trials support services include technological integration, increased outsourcing by pharmaceutical companies, and a shift towards decentralized trials to enhance efficiency and patient engagement.