Closed System Transfer Devices Market Report
Published Date: 31 January 2026 | Report Code: closed-system-transfer-devices
Closed System Transfer Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Closed System Transfer Devices market, including insights into market size, growth trends, and forecasts for the years 2023 to 2033, covering key aspects like segmentation and regional insights.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
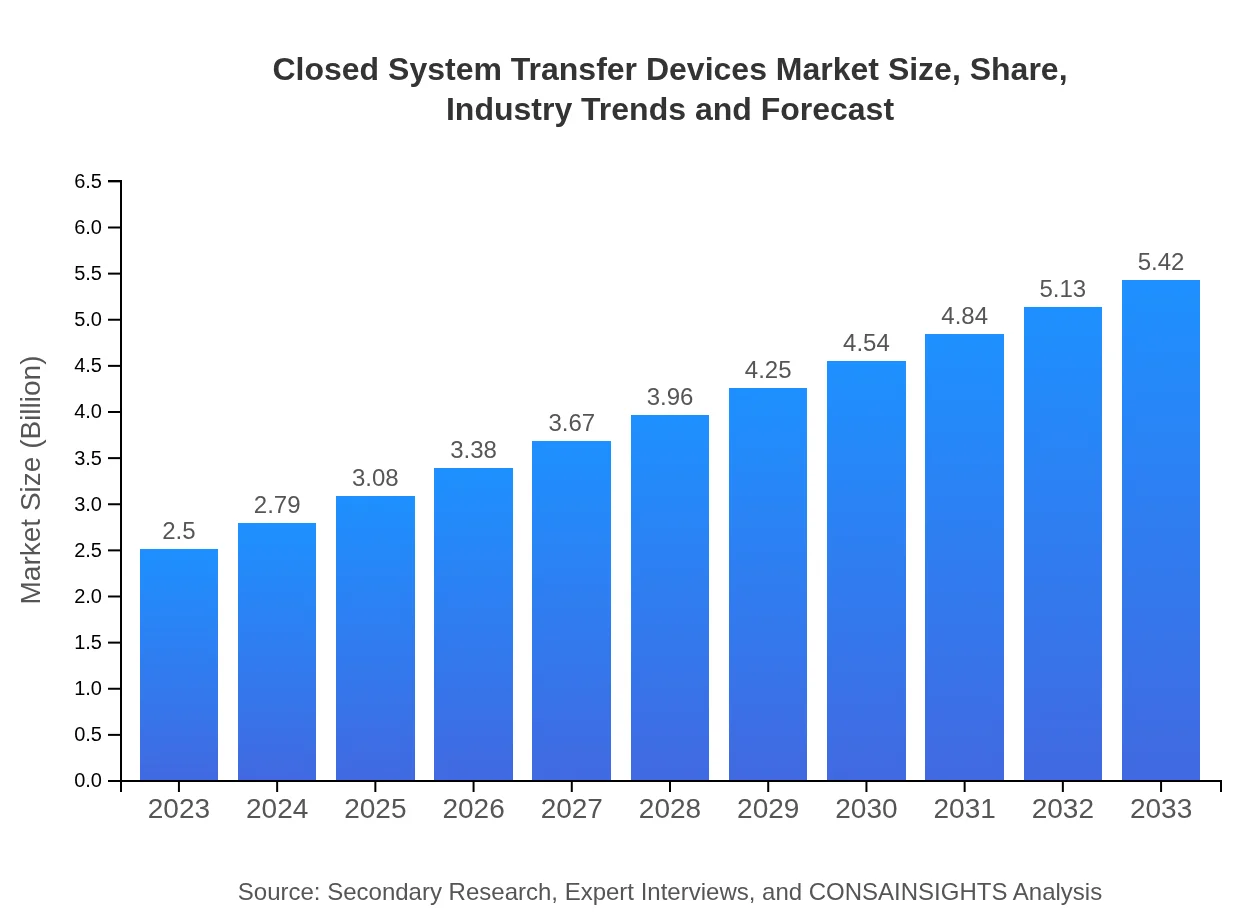
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $5.42 Billion |
| Top Companies | Baxter International Inc., B. Braun Melsungen AG, CareFusion, MediDose |
| Last Modified Date | 31 January 2026 |
Closed System Transfer Devices Market Overview
Customize Closed System Transfer Devices Market Report market research report
- ✔ Get in-depth analysis of Closed System Transfer Devices market size, growth, and forecasts.
- ✔ Understand Closed System Transfer Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Closed System Transfer Devices
What is the Market Size & CAGR of Closed System Transfer Devices market in 2023?
Closed System Transfer Devices Industry Analysis
Closed System Transfer Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Closed System Transfer Devices Market Analysis Report by Region
Europe Closed System Transfer Devices Market Report:
The European market is expected to increase from USD 0.80 billion in 2023 to USD 1.74 billion by 2033. Factors such as the aging population and a rise in the number of cytotoxic drugs contribute to this upsurge.Asia Pacific Closed System Transfer Devices Market Report:
The Asia Pacific market is projected to grow from USD 0.49 billion in 2023 to USD 1.07 billion by 2033, showing significant development in healthcare infrastructure and increasing awareness of drug safety among healthcare workers.North America Closed System Transfer Devices Market Report:
North America holds a substantial market share, anticipated to grow from USD 0.81 billion in 2023 to USD 1.76 billion by 2033. This growth is propelled by stringent healthcare regulations, high prevalence of chronic diseases, and a strong focus on drug safety among practitioners.South America Closed System Transfer Devices Market Report:
In South America, the market size is expected to expand from USD 0.05 billion in 2023 to USD 0.10 billion by 2033, influenced by rising investments in the healthcare sector and improvements in pharmaceutical regulations.Middle East & Africa Closed System Transfer Devices Market Report:
The Middle East and Africa market is estimated to grow from USD 0.35 billion in 2023 to USD 0.75 billion by 2033, driven by improvements in healthcare access and rising healthcare expenditure in these regions.Tell us your focus area and get a customized research report.
Closed System Transfer Devices Market Analysis By Product Type
The Closed System Transfer Devices market by product type reveals that passive systems dominate, expected to increase from USD 2.21 billion in 2023 to USD 4.78 billion by 2033, holding an 88.21% market share. Active systems, while smaller, are also projected to grow from USD 0.29 billion to USD 0.64 billion, capturing 11.79% of the market.
Closed System Transfer Devices Market Analysis By Application
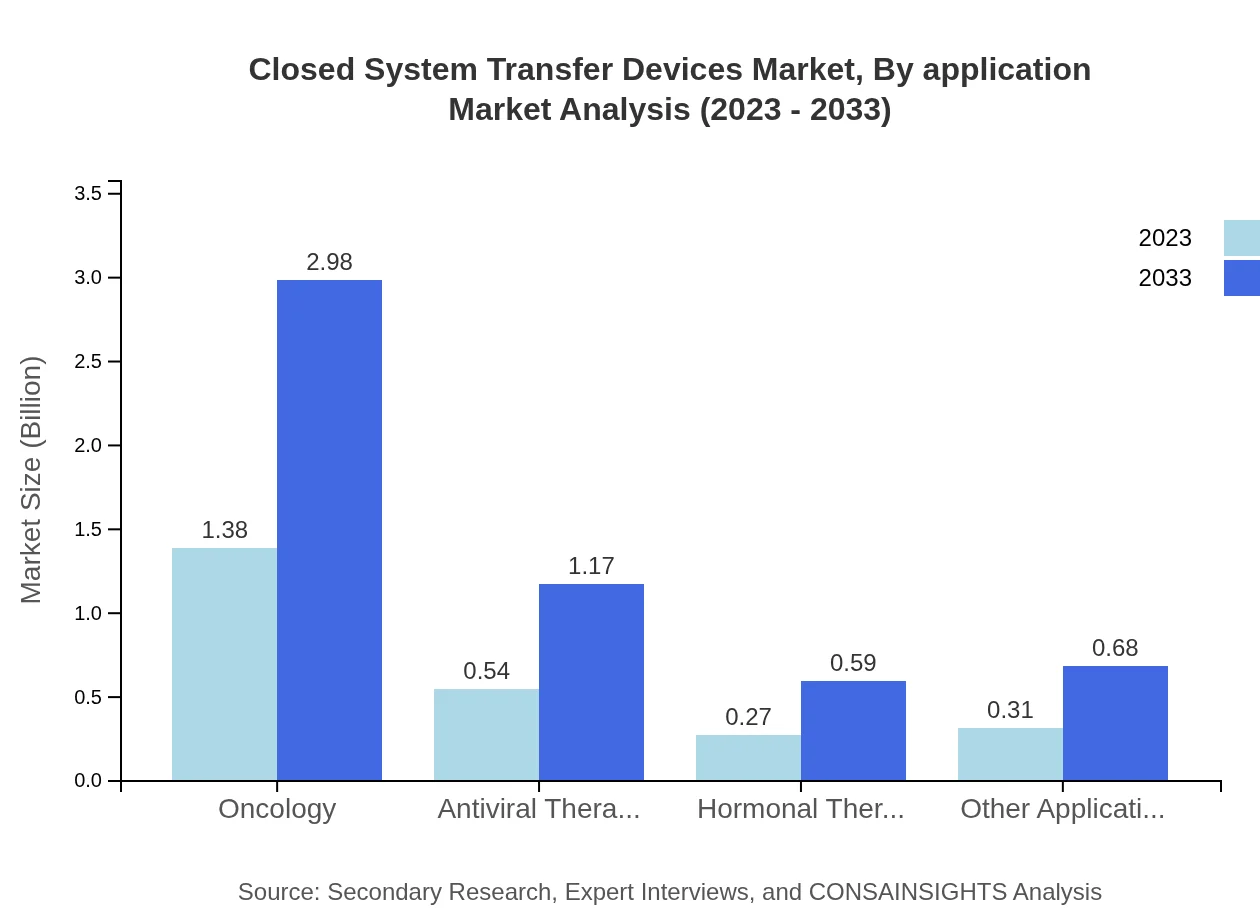
By application, oncology remains the largest segment, predicted to grow from USD 1.38 billion in 2023 to USD 2.98 billion by 2033, with a steady market share of 55.06%. Other segments, including antiviral and hormonal therapies, will see growth, but oncology is expected to remain the leader due to increasing cancer cases.
Closed System Transfer Devices Market Analysis By End User
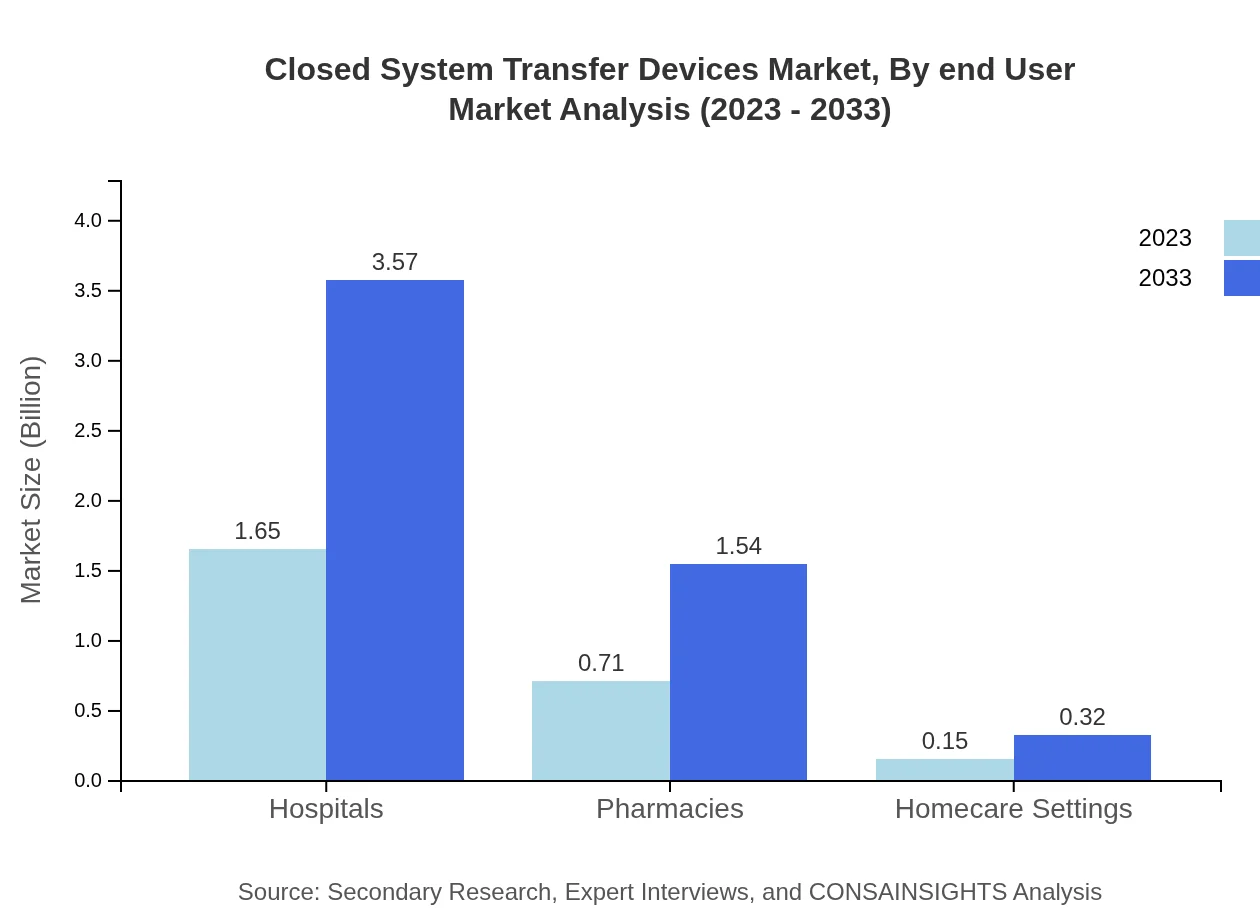
Hospitals are the largest end-user segment in the Closed System Transfer Devices market, anticipated to grow from USD 1.65 billion in 2023 to USD 3.57 billion by 2033, maintaining a consistent 65.83% market share. Pharmacies and homecare settings are also important, with expected growth in their respective segments.
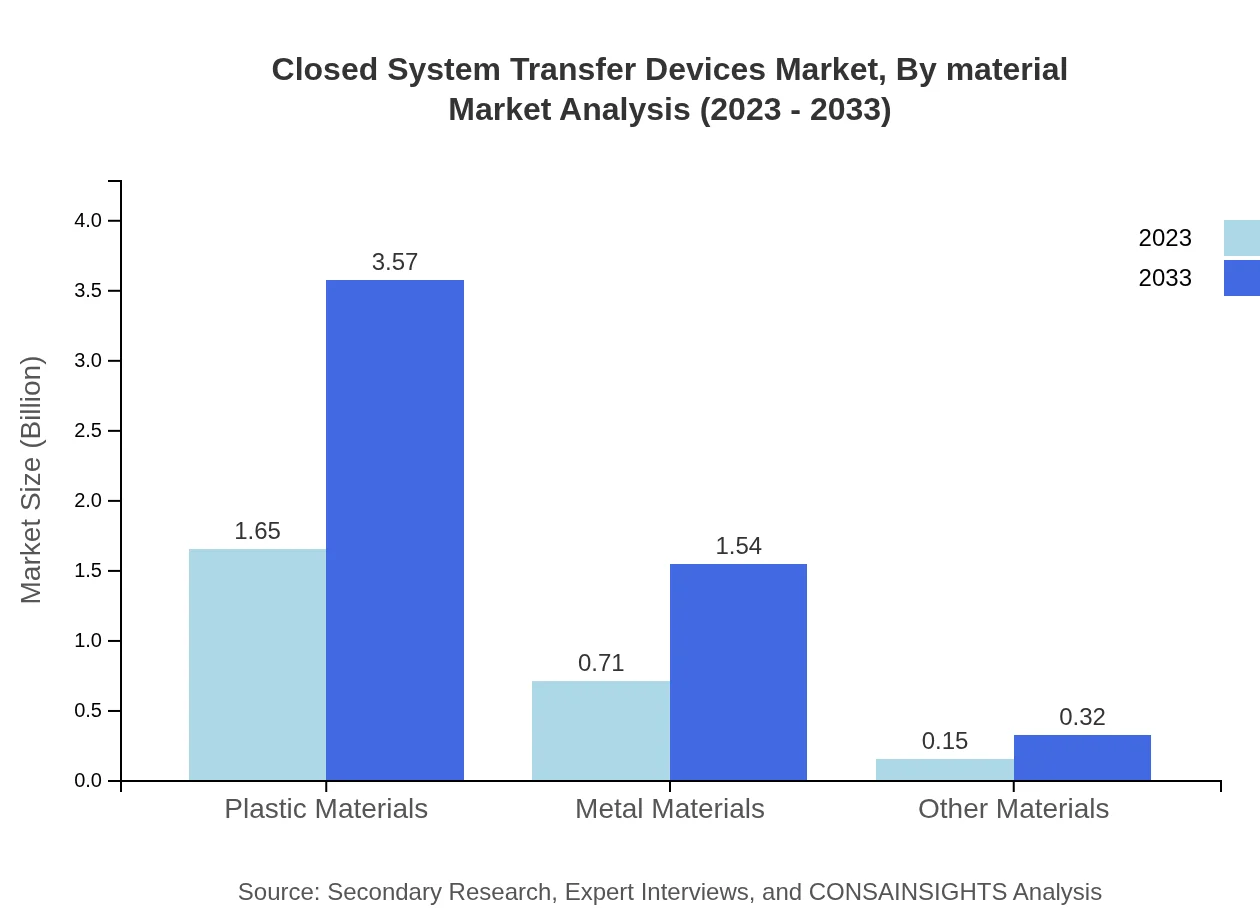
Closed System Transfer Devices Market Analysis By Material
The materials segment shows plastic materials leading the market, projected to grow from USD 1.65 billion in 2023 to USD 3.57 billion by 2033, keeping the same market share of 65.83%. Metal materials and other materials are smaller segments but are growing as manufacturers explore various applications.
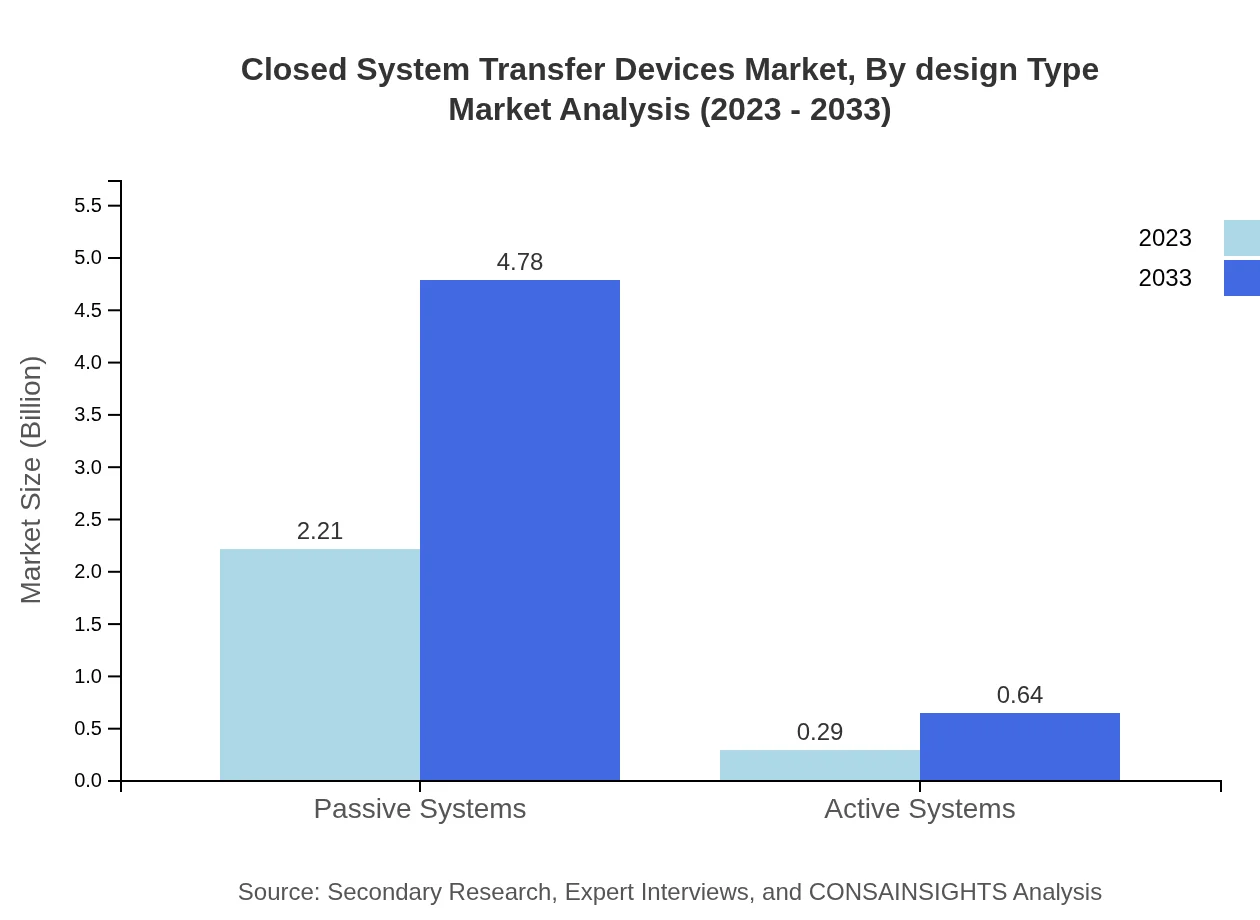
Closed System Transfer Devices Market Analysis By Design Type
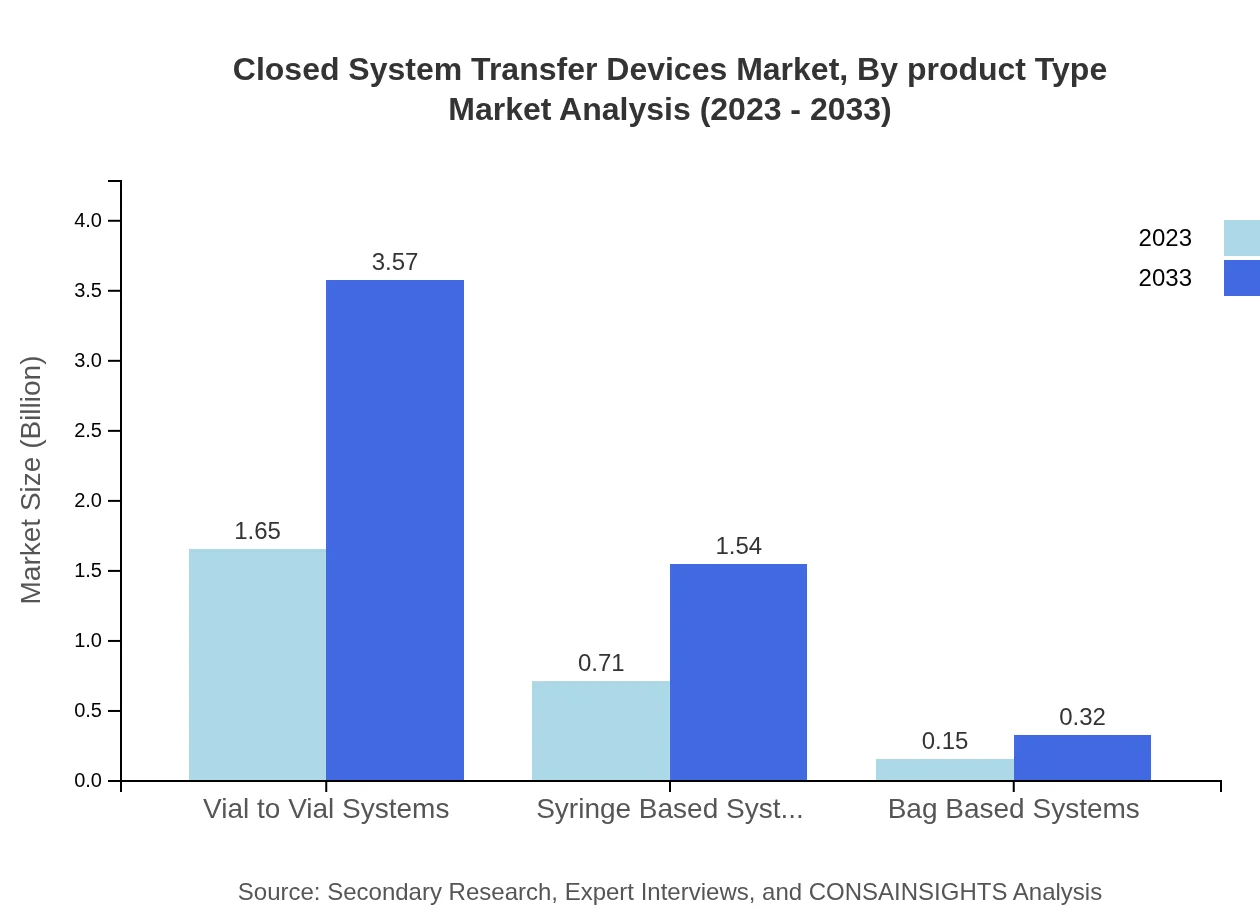
Design type segmentation reveals that vial-to-vial systems are expected to maintain a large share, growing from USD 1.65 billion in 2023 to USD 3.57 billion by 2033 (65.83% share). Syringe based systems also represent a significant market, projected to grow from USD 0.71 billion to USD 1.54 billion.
Closed System Transfer Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Closed System Transfer Devices Industry
Baxter International Inc.:
Baxter is a renowned healthcare company specializing in products that address complex medical needs, including advanced CSTDs that enhance safety in drug delivery.B. Braun Melsungen AG:
B. Braun is a global leader in medical devices and solutions, focusing on closed systems for drug delivery, ensuring quality and safety in patient care.CareFusion:
CareFusion specializes in innovative healthcare technologies, providing CSTDs that ensure safe handling of hazardous drugs, significantly contributing to overall healthcare safety.MediDose:
MediDose is known for its commitment to medication safety, producing CSTDs aimed at minimizing risks associated with drug administration and protecting healthcare workers.We're grateful to work with incredible clients.









FAQs
What is the market size of Closed-System Transfer Devices?
The global Closed-System Transfer Devices market is valued at approximately $2.5 billion in 2023 and is expected to grow at a CAGR of 7.8%, reaching projected market size figures for 2033 that reflect continued expansion.
What are the key market players or companies in the Closed-System Transfer Devices industry?
Key players in the Closed-System Transfer Devices market include leading companies that specialize in oncology and safety medical products. These organizations drive innovation and provide competitive solutions that enhance safety for healthcare professionals.
What are the primary factors driving the growth in the Closed-System Transfer Devices industry?
The growth of the Closed-System Transfer Devices industry is driven by increasing safety concerns in drug handling, growing incidence of cancer therapies, and regulatory mandates for patient protection. These factors create a robust market demand.
Which region is the fastest Growing in the Closed-System Transfer Devices?
The fastest-growing region for Closed-System Transfer Devices is Europe, with market expansion from $0.80 billion in 2023 to $1.74 billion by 2033. Other regions, like Asia-Pacific, also show significant growth potential.
Does ConsaInsights provide customized market report data for the Closed-System Transfer Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the Closed-System Transfer Devices sector, catering to unique client requirements for deeper insights and strategic planning.
What deliverables can I expect from this Closed-System Transfer Devices market research project?
Expect comprehensive deliverables, including detailed market analysis, forecasts, regional and segment data, industry trends, and actionable insights tailored for stakeholders in the Closed-System Transfer Devices market.
What are the market trends of Closed-System Transfer Devices?
Market trends in Closed-System Transfer Devices highlight a shift towards passive systems, increasing adoption in hospitals and homecare settings, and a growing focus on safety regarding handling hazardous drugs.