Clostridium Difficile Infection Market Report
Published Date: 31 January 2026 | Report Code: clostridium-difficile-infection
Clostridium Difficile Infection Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the market for Clostridium Difficile Infection (CDI) from 2023 to 2033. It includes insights on market size, CAGR, regional analysis, industry trends, and leading players, offering a comprehensive view of future growth prospects and challenges.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
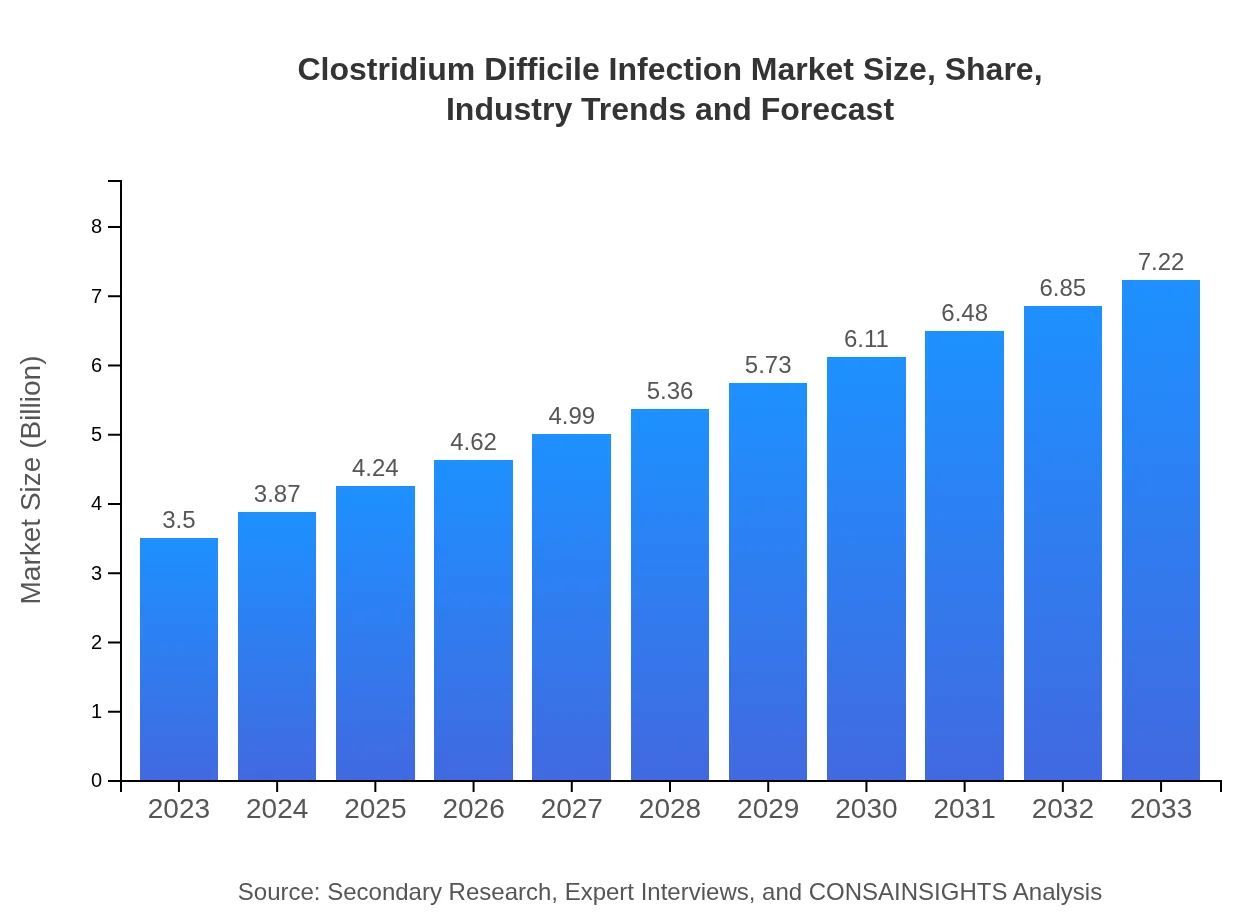
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.3% |
| 2033 Market Size | $7.22 Billion |
| Top Companies | AstraZeneca, Pfizer Inc., Viropharma, Merck & Co., Boehringer Ingelheim |
| Last Modified Date | 31 January 2026 |
Clostridium Difficile Infection Market Overview
Customize Clostridium Difficile Infection Market Report market research report
- ✔ Get in-depth analysis of Clostridium Difficile Infection market size, growth, and forecasts.
- ✔ Understand Clostridium Difficile Infection's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clostridium Difficile Infection
What is the Market Size & CAGR of Clostridium Difficile Infection market in 2033?
Clostridium Difficile Infection Industry Analysis
Clostridium Difficile Infection Market Segmentation and Scope
Tell us your focus area and get a customized research report.
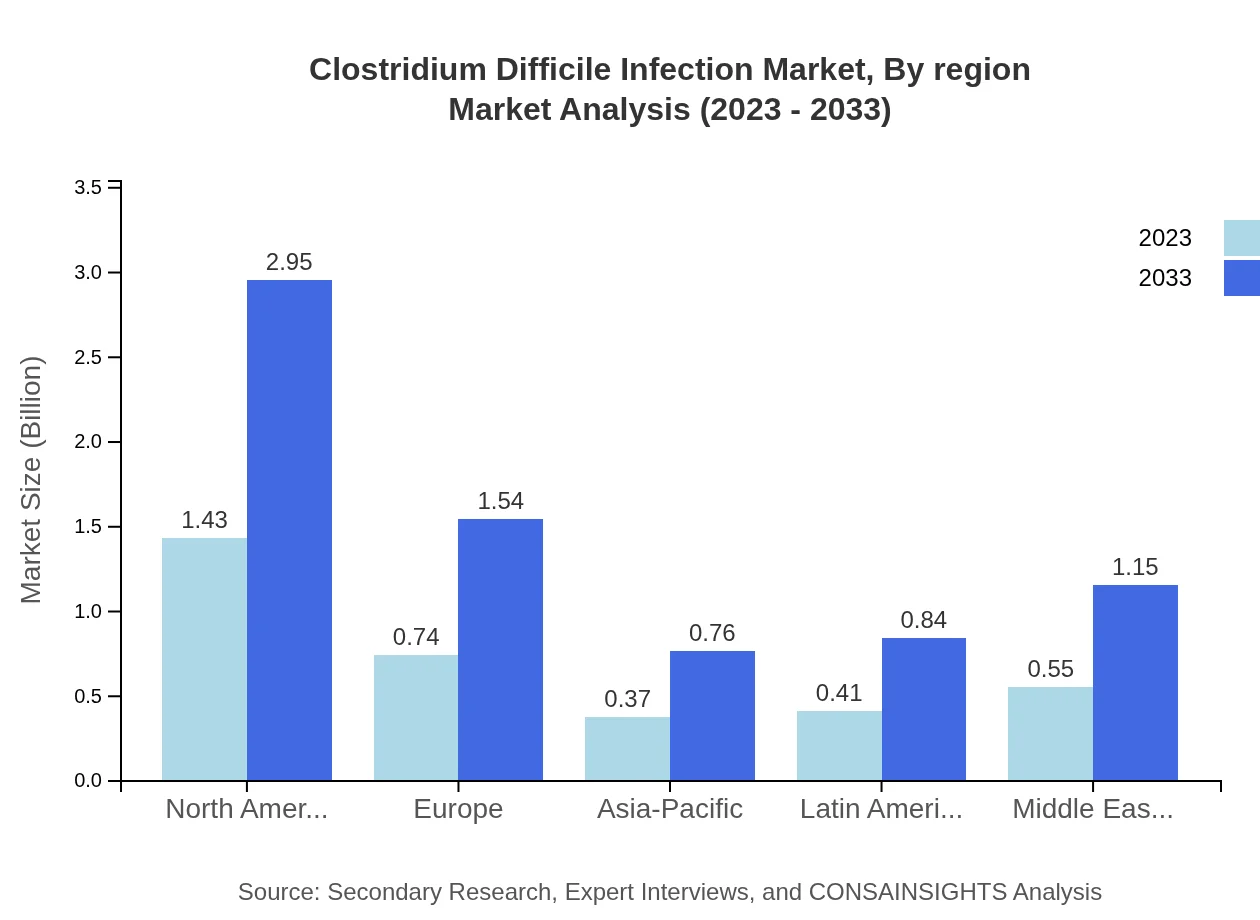
Clostridium Difficile Infection Market Analysis Report by Region
Europe Clostridium Difficile Infection Market Report:
The European CDI market is anticipated to grow from $1.16 billion in 2023 to $2.39 billion by 2033. Increased adoption of advanced diagnostic methods, high healthcare spending, and a growing geriatric population are integral market drivers. Countries such as Germany and the UK are leading in terms of market share due to established healthcare systems.Asia Pacific Clostridium Difficile Infection Market Report:
In the Asia Pacific region, the market is anticipated to grow from $0.54 billion in 2023 to $1.12 billion by 2033. Factors such as rising healthcare expenditure, increased prevalence of hospital-acquired infections, and improved healthcare infrastructure drive this growth. Countries like Japan and Australia are witnessing advancements in CDI management practices, creating ample market opportunities.North America Clostridium Difficile Infection Market Report:
North America holds the largest market share, with a projected size of $1.28 billion in 2023 and expected to rise to $2.64 billion by 2033. The robust healthcare infrastructure, increased focus on infection control, and high prevalence of CDI cases significantly contribute to this growth. The USA is the primary market, leading in both treatment options and diagnostic capabilities.South America Clostridium Difficile Infection Market Report:
The South American CDI market is expected to expand from $0.30 billion in 2023 to $0.62 billion by 2033, driven primarily by rising awareness and the growing burden of infectious diseases. Enhanced healthcare policies and government funding for infectious disease management are crucial factors supporting market development in this region.Middle East & Africa Clostridium Difficile Infection Market Report:
In the Middle East and Africa, the CDI market is projected to reach $0.45 billion by 2033, growing from $0.22 billion in 2023. Improved healthcare accessibility, rising awareness of CDI treatment, and preventative measures are contributing factors, particularly in GCC countries.Tell us your focus area and get a customized research report.
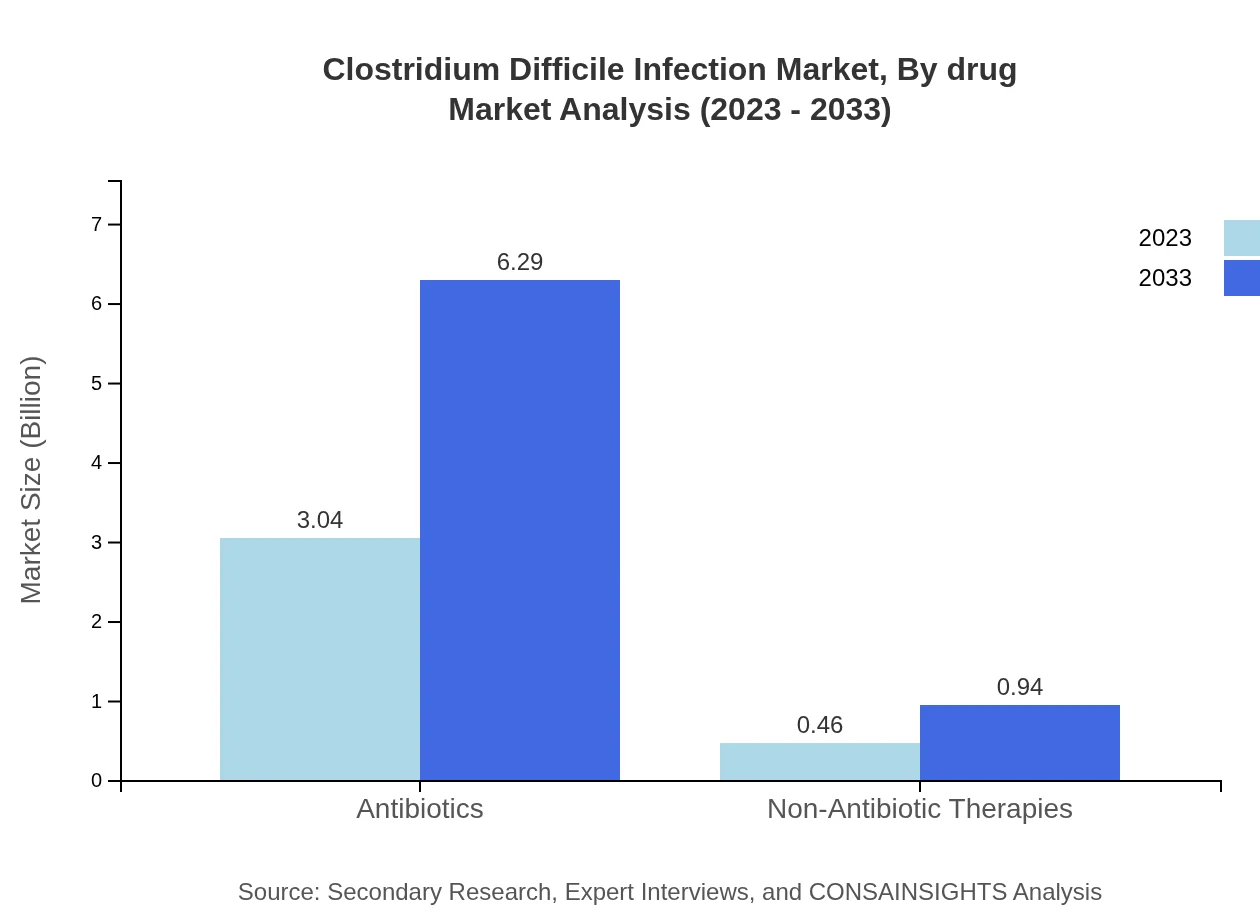
Clostridium Difficile Infection Market Analysis By Drug
In the drug segment, antibiotics are anticipated to significantly dominate the CDI treatment market, projected to grow from $3.04 billion in 2023 to $6.29 billion by 2033, holding a market share of 87% throughout. Non-antibiotic therapies, while currently smaller, are also expected to expand from $0.46 billion in 2023 to $0.94 billion by 2033, achieving a 13% market share.
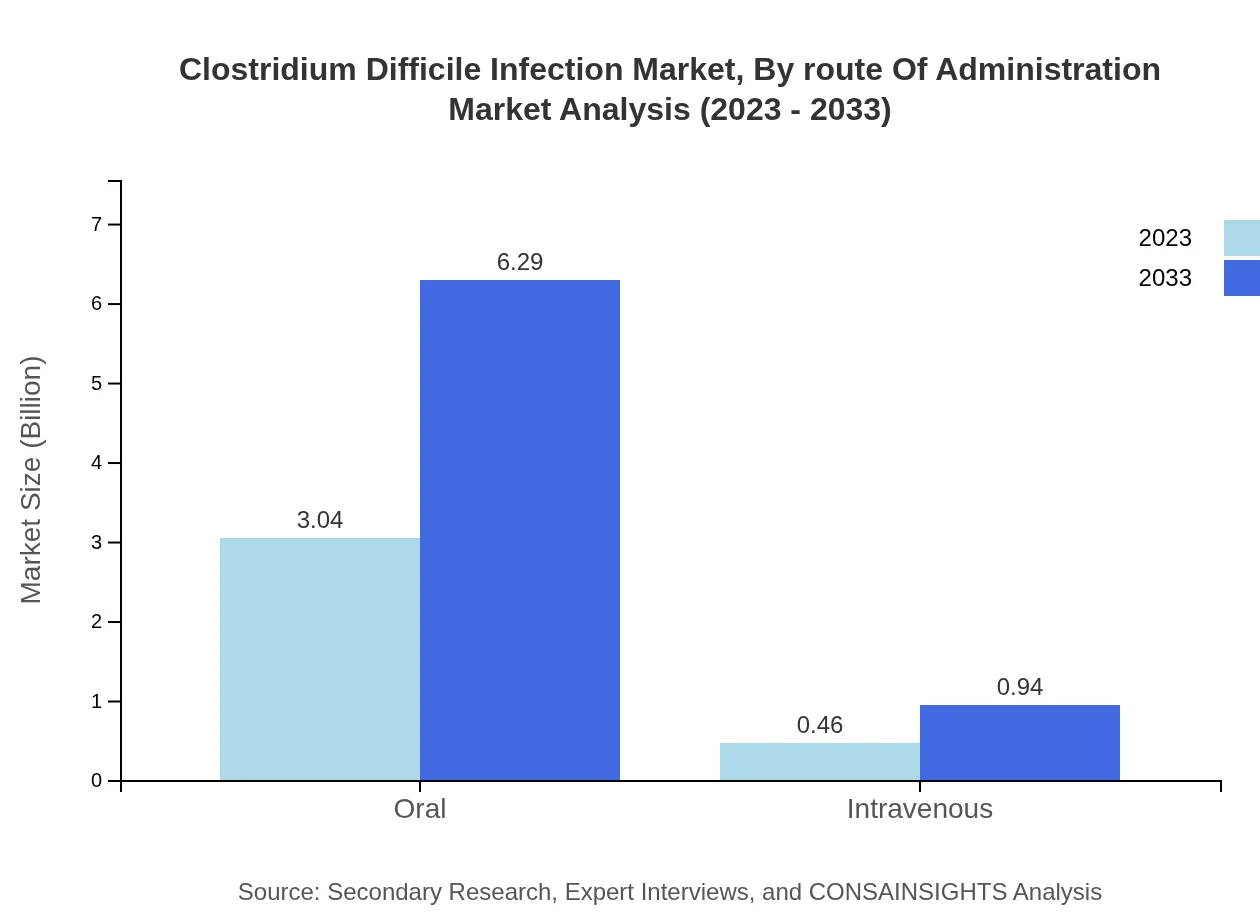
Clostridium Difficile Infection Market Analysis By Route Of Administration
Oral administration remains the primary method for CDI treatments, accounting for approximately 87% of the market, projected to rise from $3.04 billion in 2023 to $6.29 billion by 2033. Meanwhile, intravenous routes are anticipated to capture a smaller share, growing from $0.46 billion in 2023 to $0.94 billion, representing 13% of the market share.
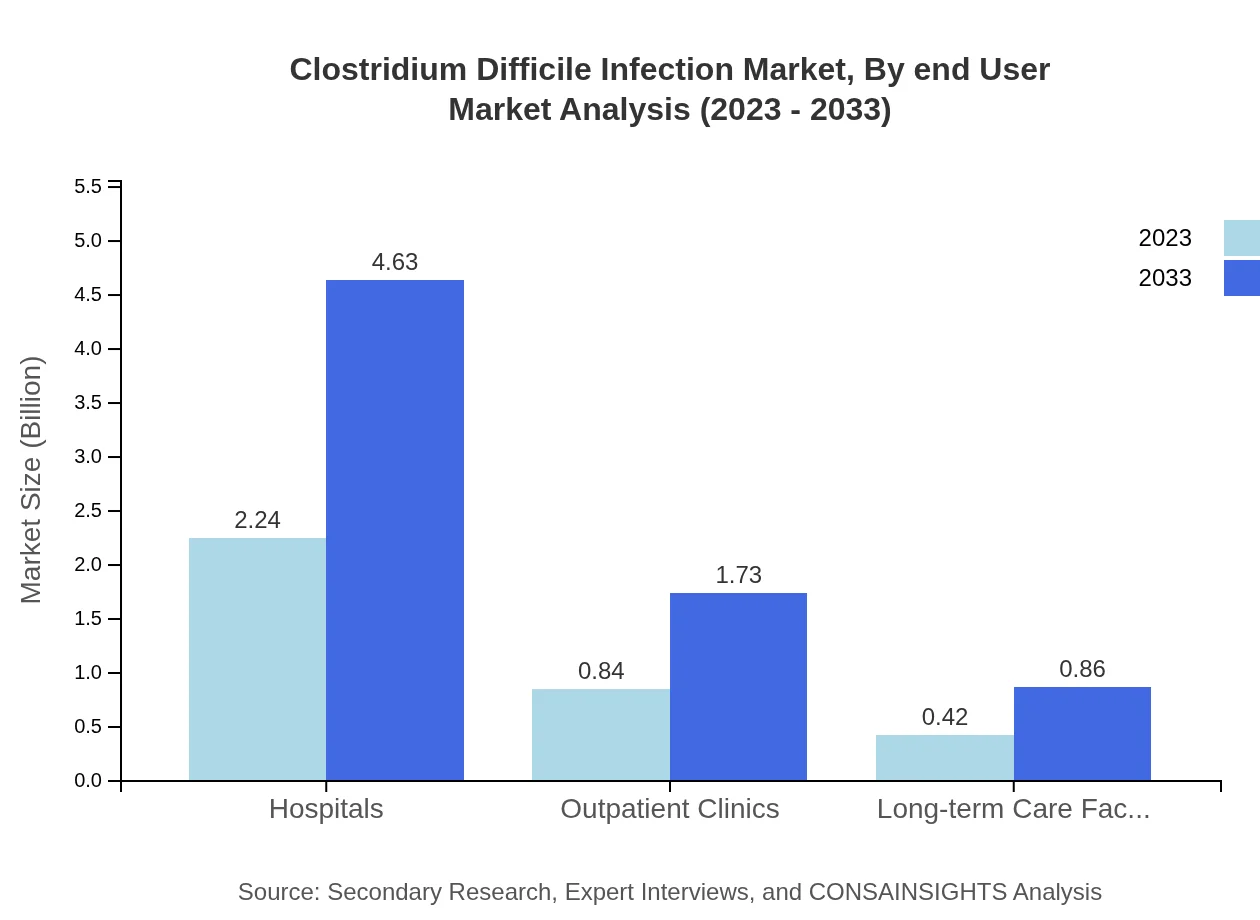
Clostridium Difficile Infection Market Analysis By End User
Hospitals are the largest end-user segment in the CDI market, expected to grow from $2.24 billion in 2023 to $4.63 billion by 2033, with a share of 64%. Outpatient clinics follow with a market size growth from $0.84 billion to $1.73 billion, representing a 24% share. Long-term care facilities will also see a notable increase in their segment from $0.42 billion to $0.86 billion.
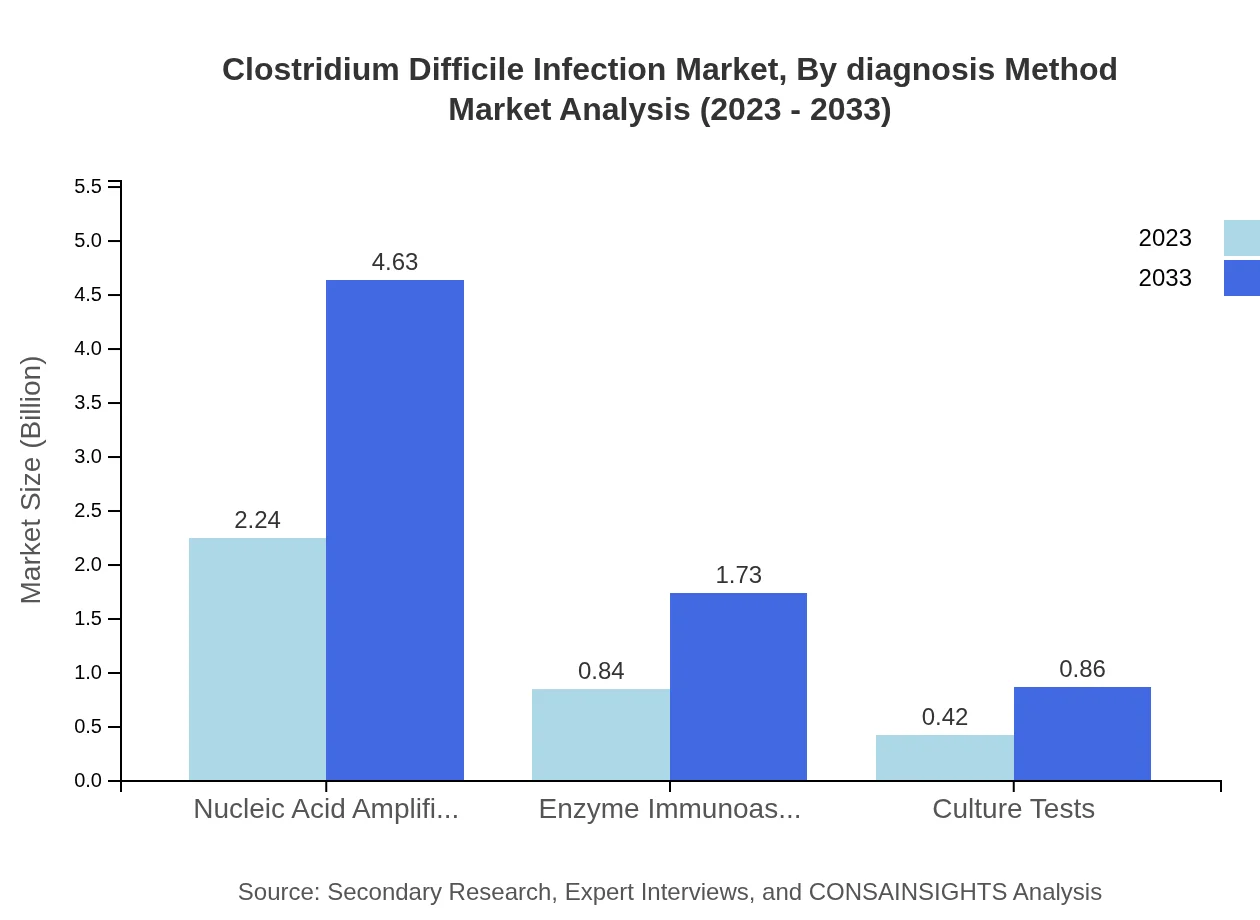
Clostridium Difficile Infection Market Analysis By Diagnosis Method
Nucleic acid amplification tests lead the CDI diagnosis segment, expected to grow from $2.24 billion in 2023 to $4.63 billion by 2033, capturing 64% market share. Enzyme immunoassays and culture tests will have smaller shares, growing from $0.84 billion and $0.42 billion respectively.
Clostridium Difficile Infection Market Analysis By Region
Regional analysis highlights North America as the dominant market, followed by Europe and Asia Pacific. Each region presents unique opportunities for market growth influenced by factors such as healthcare infrastructure, government policies, and population health dynamics. This segmentation guide helps stakeholders tailor their strategies effectively based on regional needs.
Clostridium Difficile Infection Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clostridium Difficile Infection Industry
AstraZeneca:
AstraZeneca is a major player in developing antibiotics targeting CDI, continually working towards innovations in antibiotic therapies.Pfizer Inc.:
Pfizer actively invests in novel CDI treatment development, focusing on both antibiotics and non-antibiotic therapeutic options.Viropharma:
A company known for its FDA-approved treatments for CDI, focusing on advanced therapeutic solutions.Merck & Co.:
Merck showcases a strong portfolio in infectious disease management, including significant contributions to the CDI market.Boehringer Ingelheim:
This biotech company invests heavily in research and development for CDI therapies, leading to innovative solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of clostridium Difficile Infection?
The global market size for Clostridium Difficile Infection was valued at approximately $3.5 billion in 2023, with an anticipated compound annual growth rate (CAGR) of 7.3%, indicating robust growth in the coming years as awareness and treatment options expand.
What are the key market players or companies in this clostridium Difficile Infection industry?
Key players in the Clostridium Difficile Infection market include major pharmaceutical companies and biotechnology firms focused on antibiotic development and non-antibiotic therapies, each contributing to innovative solutions to combat the rising infection rates.
What are the primary factors driving the growth in the clostridium Difficile Infection industry?
Factors driving growth in this industry include increasing incidence of infections, advancement in diagnostic technologies, growing awareness of infectious diseases, and enhanced funding for research and development of treatment options and preventive measures.
Which region is the fastest Growing in the clostridium Difficile Infection?
The Asia-Pacific region is the fastest-growing market for Clostridium Difficile Infection, projecting growth from $0.54 billion in 2023 to $1.12 billion by 2033, as healthcare infrastructure improves and awareness of infection risks increases.
Does ConsaInsights provide customized market report data for the clostridium Difficile Infection industry?
Yes, ConsaInsights offers customized market reports tailored to specific client needs, allowing businesses to access unique insights and data relevant to the Clostridium Difficile Infection market, enhancing strategic decision-making.
What deliverables can I expect from this clostridium Difficile Infection market research project?
Deliverables include comprehensive market analysis reports, trend insights, competitive landscape summaries, regional performance evaluations, and forecasts for multiple segments, ensuring that stakeholders receive actionable and detailed information.
What are the market trends of clostridium Difficile Infection?
Current trends in the Clostridium Difficile Infection market involve a shift towards non-antibiotic therapies, increased focus on infection prevention strategies, and the adoption of advanced diagnostic tests to improve timely treatment and management of infections.