Clot Management Devices Market Report
Published Date: 31 January 2026 | Report Code: clot-management-devices
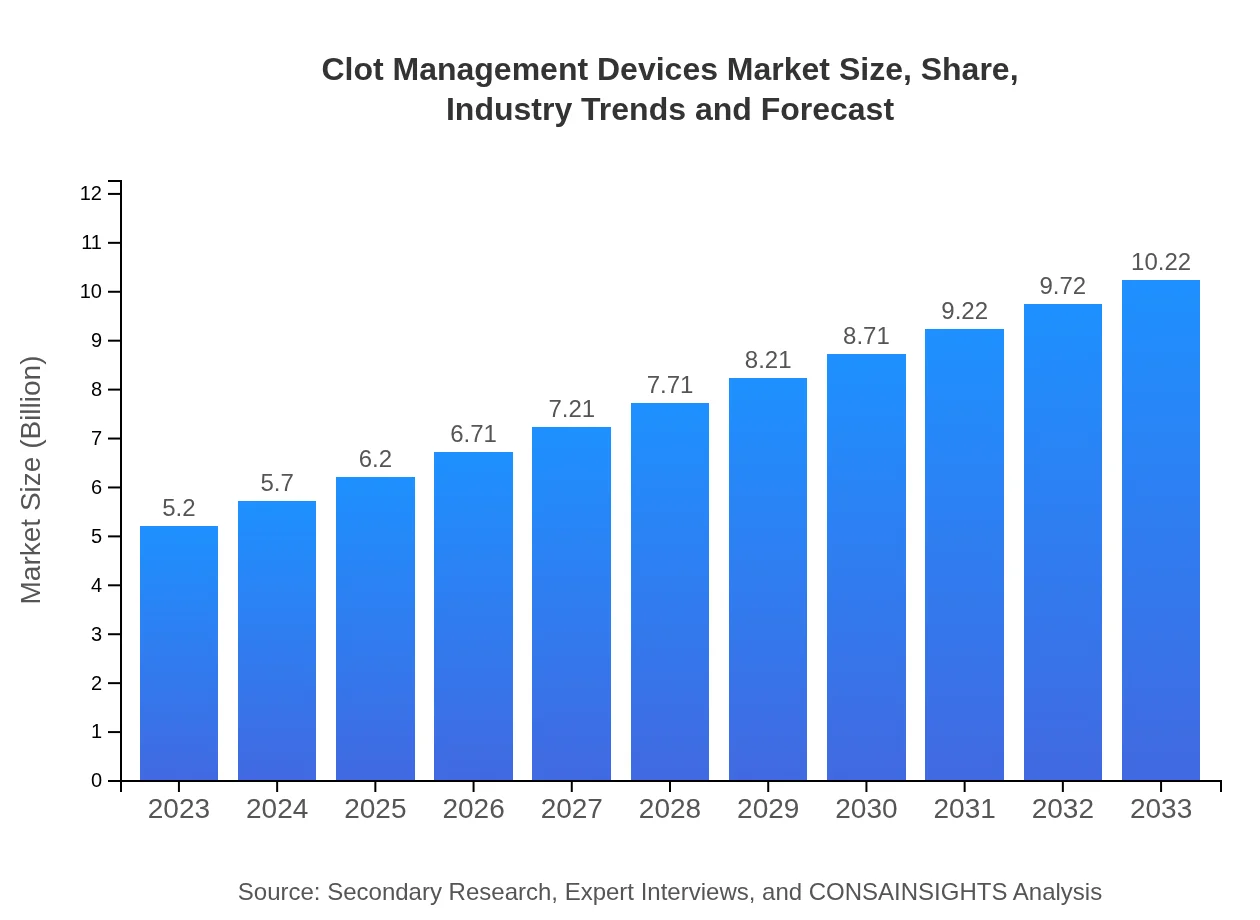
Clot Management Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Clot Management Devices market, covering market size, trends, key players, and forecasts from 2023 to 2033 to offer insights into future growth opportunities and challenges.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Medtronic , Boston Scientific, Terumo Corporation, Abbott Laboratories, Philips Healthcare |
| Last Modified Date | 31 January 2026 |
Clot Management Devices Market Overview
Customize Clot Management Devices Market Report market research report
- ✔ Get in-depth analysis of Clot Management Devices market size, growth, and forecasts.
- ✔ Understand Clot Management Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Clot Management Devices
What is the Market Size & CAGR of Clot Management Devices market in 2023?
Clot Management Devices Industry Analysis
Clot Management Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Clot Management Devices Market Analysis Report by Region
Europe Clot Management Devices Market Report:
In Europe, the market is expected to rise from $1.31 billion in 2023 to $2.58 billion by 2033, driven by a robust healthcare system, an aging population, and growing patient awareness about preventive treatment options.Asia Pacific Clot Management Devices Market Report:
In the Asia Pacific region, the Clot Management Devices market is expected to grow from $1.02 billion in 2023 to $2.01 billion by 2033, reflecting a significant CAGR driven by increasing healthcare infrastructure investments and rising awareness regarding cardiovascular diseases.North America Clot Management Devices Market Report:
North America is poised to maintain its leadership with a market size of $1.71 billion in 2023, growing to $3.35 billion by 2033. Factors include advanced healthcare offerings, a high prevalence of chronic diseases, and strong R&D activities among leading players.South America Clot Management Devices Market Report:
The South American market is anticipated to increase from $0.50 billion in 2023 to $0.99 billion in 2033. Factors such as enhancing healthcare services and growing incidences of clot-related disorders are promoting market growth in this region.Middle East & Africa Clot Management Devices Market Report:
The Middle East and Africa's market is expected to grow from $0.66 billion in 2023 to $1.30 billion by 2033. Increased healthcare investments and a heightened focus on improving cardiovascular health contribute to this growth trajectory.Tell us your focus area and get a customized research report.
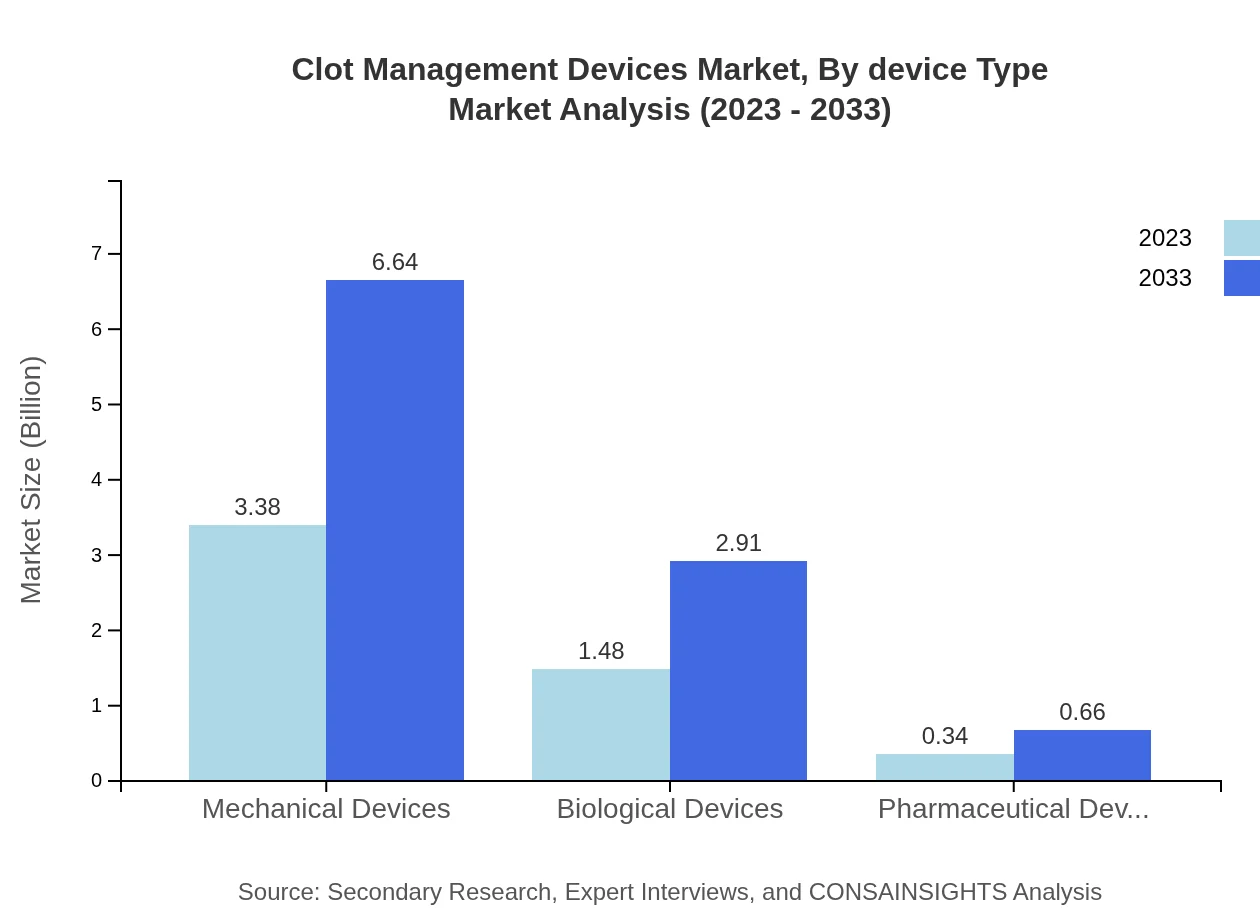
Clot Management Devices Market Analysis By Device Type
The device type segment illustrates substantial growth, with mechanical devices generating the largest revenue share, maintaining a consistent 65% market share throughout the forecast period. Biological devices and pharmaceutical devices represent significant growth areas, accounting for 28.51% each in 2023 and anticipated to remain constant through 2033.
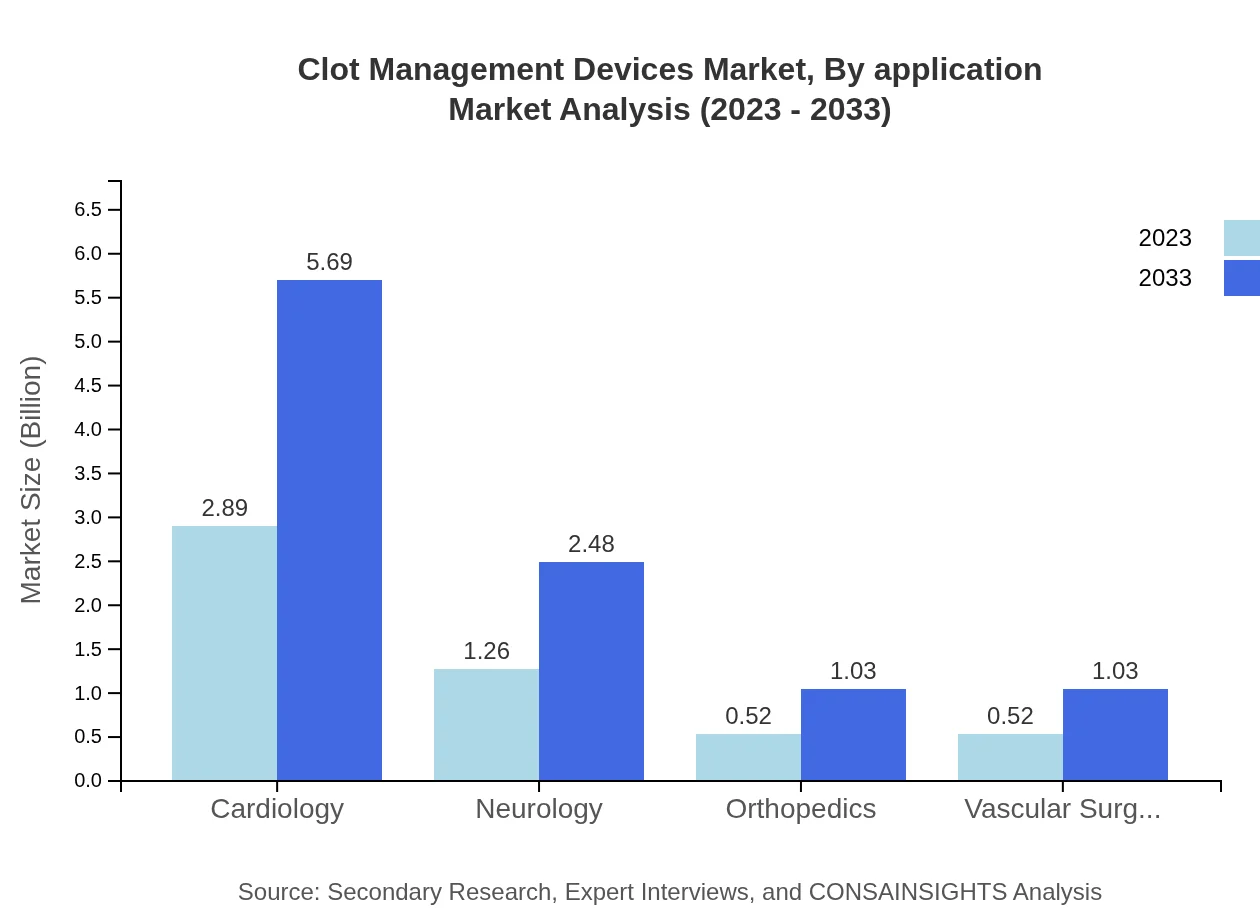
Clot Management Devices Market Analysis By Application
By application, cardiology holds a substantial portion of the market with a projected growth from $2.89 billion in 2023 to $5.69 billion in 2033. Neurology follows at $1.26 billion and directly correlates to improved treatments and technologies being adopted. Orthopedics and vascular surgery also show similar growth patterns.
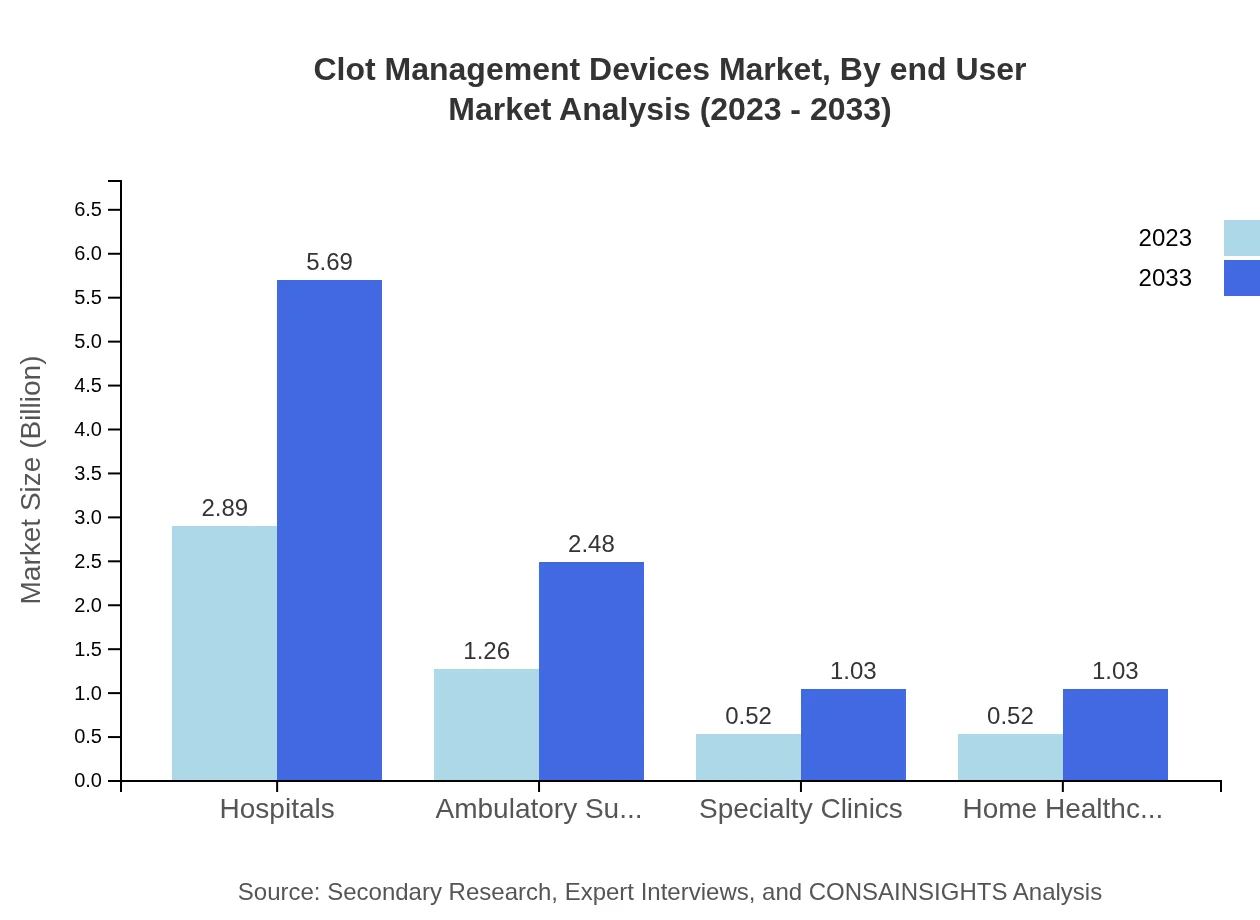
Clot Management Devices Market Analysis By End User
Hospitals dominate the end-user market, sharing approximately 55.63% of the market in both 2023 and 2033, primarily due to their high patient turnover and extensive use of clot management devices. Ambulatory surgical centers and specialty clinics are also significant contributors.
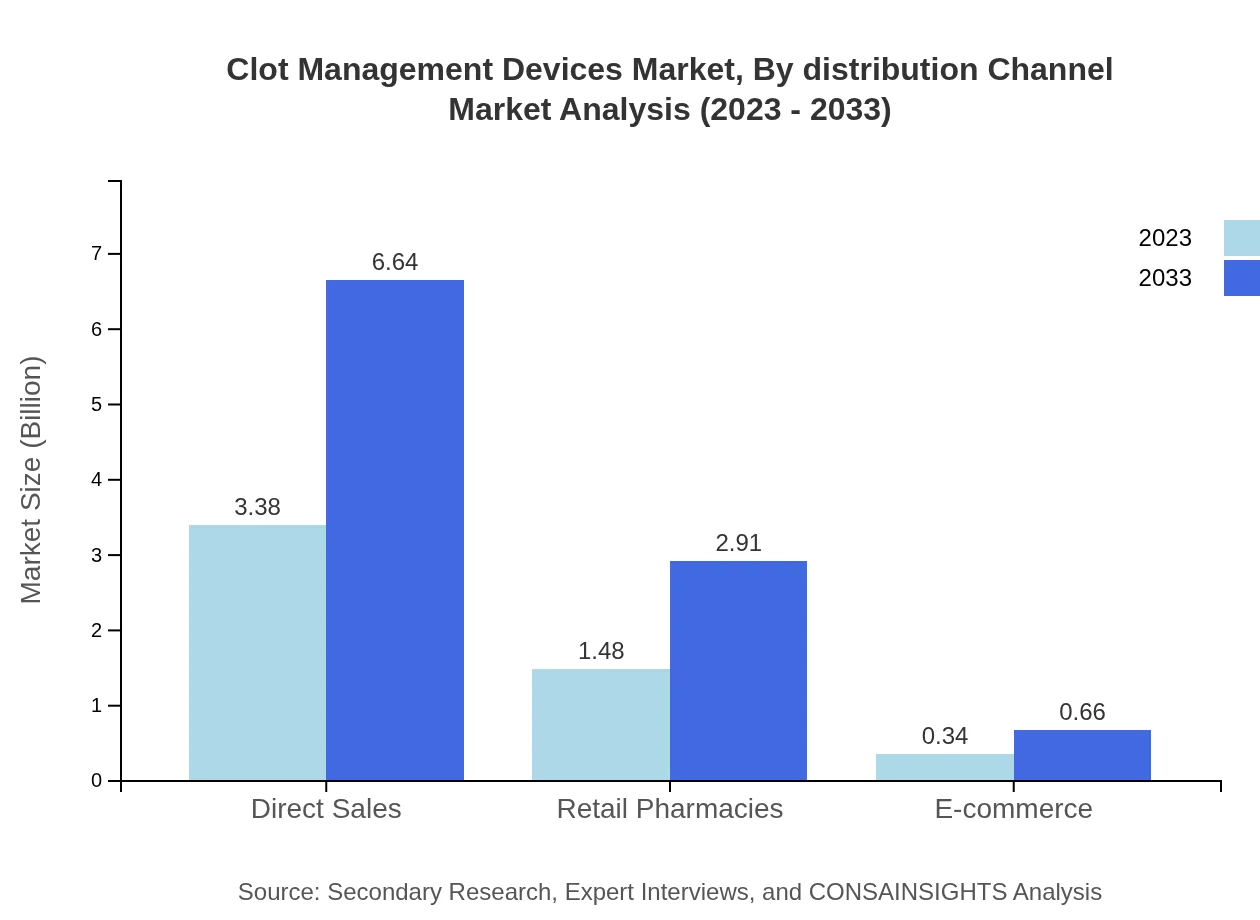
Clot Management Devices Market Analysis By Distribution Channel
Distribution channels such as direct sales and retail pharmacies contribute significantly, accounting for 65% and 28.51% shares in 2023, respectively. E-commerce shows modest growth potential due to the increasing consumer tendencies toward online healthcare solutions.
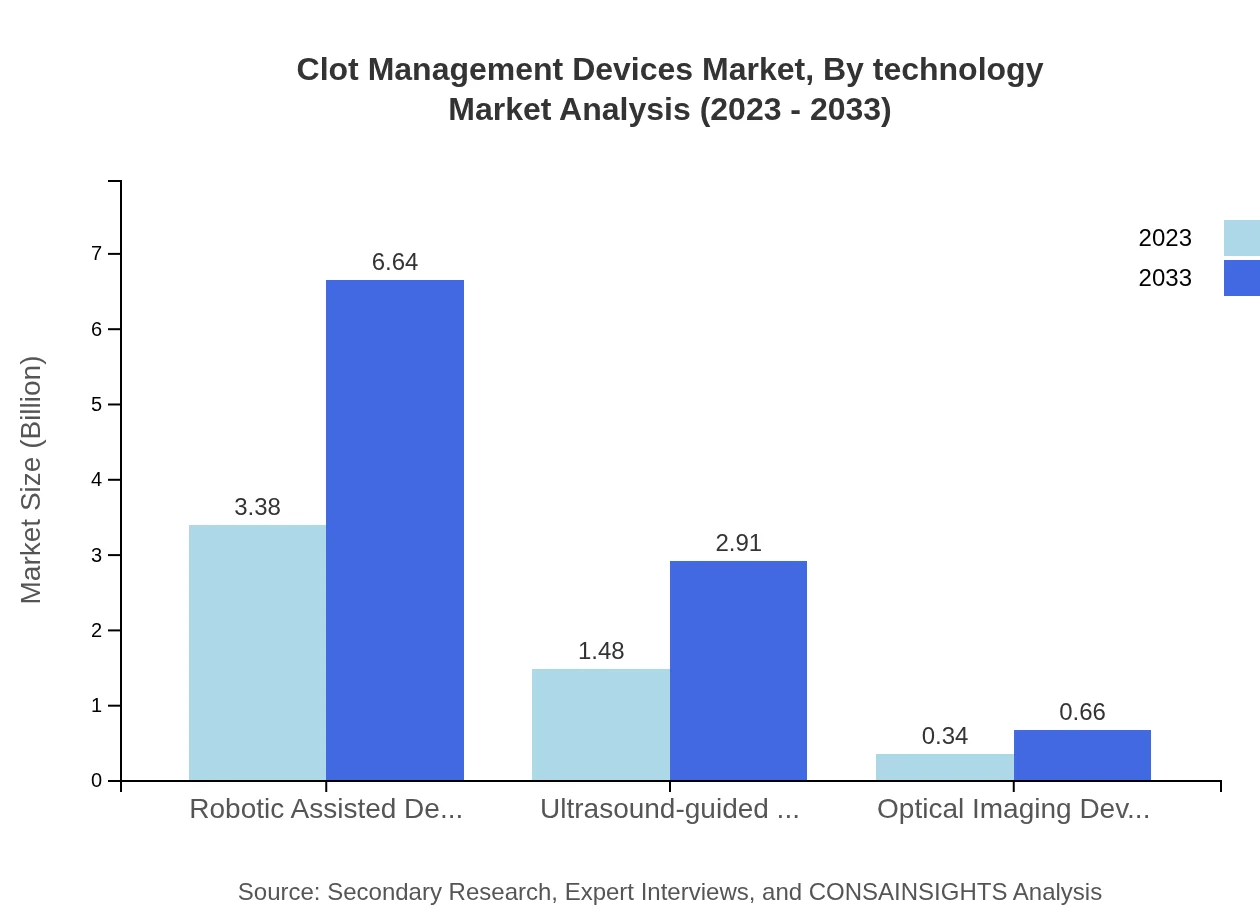
Clot Management Devices Market Analysis By Technology
Technological advancements propel the Clot Management Devices market forward, with robotic-assisted devices emerging as frontrunners, representing a 65% share in 2023 and projected to continue this trend. Innovations in ultrasound-guided devices also show promise, expanding rapidly from $1.48 billion in 2023 to $2.91 billion by 2033.
Clot Management Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Clot Management Devices Industry
Medtronic :
A leading player providing advanced clot management solutions through innovative technologies and comprehensive surgical devices.Boston Scientific:
Boston Scientific specializes in developing medical devices used for interventions in clot management, emphasizing precision and effectiveness.Terumo Corporation:
Known for its wide array of medical devices, Terumo Corporation integrates advanced technology in its clot management devices to enhance patient care.Abbott Laboratories:
Abbott provides innovative technologies for minimal invasive surgeries, improving patient outcomes significantly within the clot management domain.Philips Healthcare:
Focusing on health technology innovation, Philips offers integrated solutions for diagnosing and treating clots, targeting improved operational efficiencies.We're grateful to work with incredible clients.









FAQs
What is the market size of clot Management Devices?
The global clot management devices market is valued at approximately $5.2 billion in 2023 and is projected to grow at a CAGR of 6.8%, reaching notable growth up to 2033.
What are the key market players or companies in this clot Management Devices industry?
Key players in the clot management devices industry include major healthcare firms specializing in therapeutic devices, however detailed company names and their specific roles would be highlighted in full market research reports.
What are the primary factors driving the growth in the clot management devices industry?
Growth in this industry is primarily driven by the rising prevalence of cardiovascular diseases, technological advancements in medical devices, and increased healthcare expenditure focusing on improved patient care.
Which region is the fastest Growing in the clot management devices market?
North America is currently the fastest-growing region, with a market size projected to increase from $1.71 billion in 2023 to $3.35 billion by 2033, driven by robust healthcare infrastructure and increasing demand for advanced medical technologies.
Does ConsaInsights provide customized market report data for the clot management devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the clot management devices industry, allowing clients to obtain precise insights relevant to their business objectives.
What deliverables can I expect from this clot management devices market research project?
Deliverables typically include detailed market analysis reports, segmented data, trend analysis, and forecasts, alongside actionable insights aimed at enhancing final decision-making.
What are the market trends of clot management devices?
Current trends include a shift towards minimally invasive procedures, increased use of robotic-assisted devices, and innovations in imaging technologies that enhance diagnostics and treatment outcomes.