Congestive Heart Failure Chf Treatment Devices Market Report
Published Date: 31 January 2026 | Report Code: congestive-heart-failure-chf-treatment-devices
Congestive Heart Failure Chf Treatment Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Congestive Heart Failure (CHF) treatment devices market, covering key trends, market dynamics, and forecasts from 2023 to 2033. Insights into market size, growth rates, and regional breakdown provide a crucial understanding for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
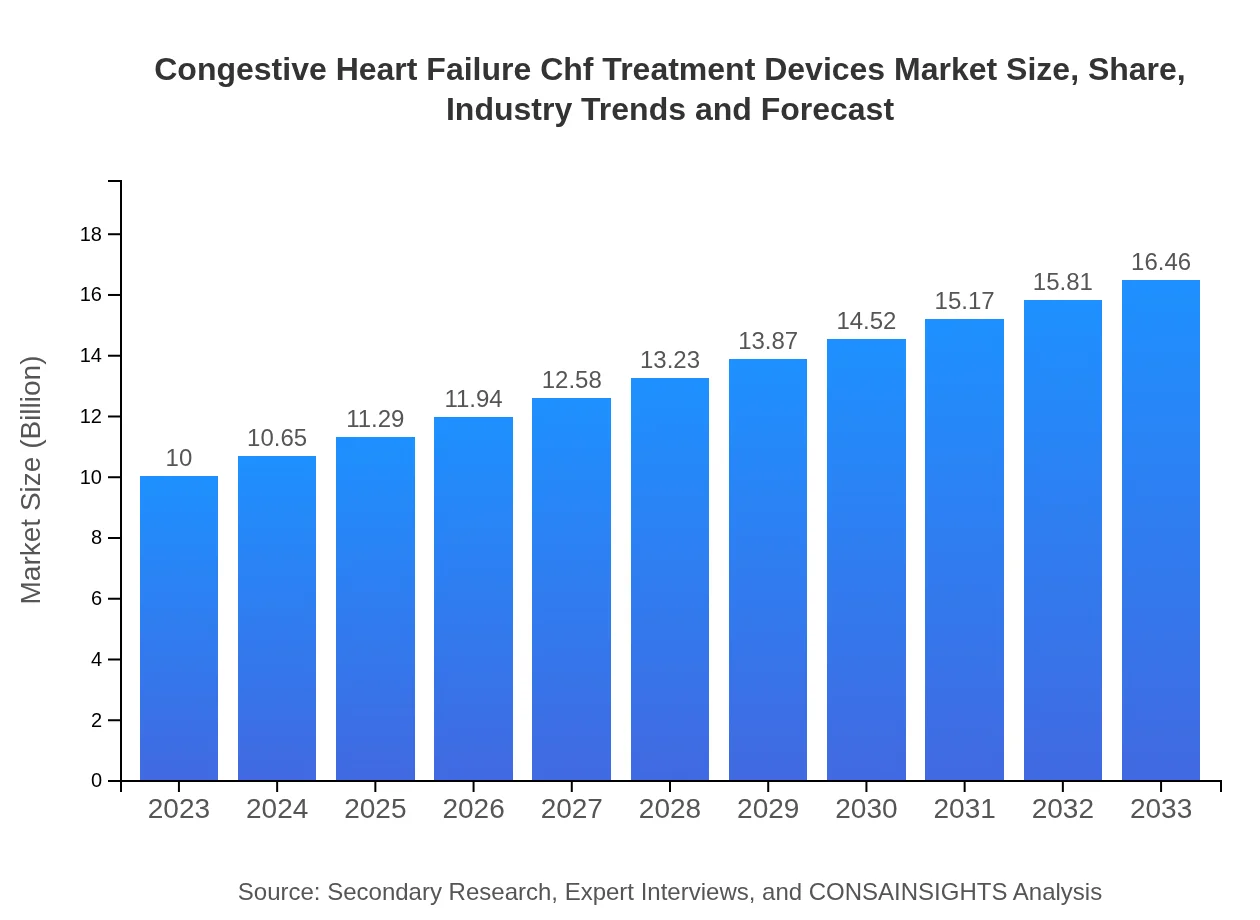
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | Medtronic , Abbott Laboratories, Boston Scientific, Biotronik, Philips Healthcare |
| Last Modified Date | 31 January 2026 |
Congestive Heart Failure Chf Treatment Devices Market Overview
Customize Congestive Heart Failure Chf Treatment Devices Market Report market research report
- ✔ Get in-depth analysis of Congestive Heart Failure Chf Treatment Devices market size, growth, and forecasts.
- ✔ Understand Congestive Heart Failure Chf Treatment Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Congestive Heart Failure Chf Treatment Devices
What is the Market Size & CAGR of Congestive Heart Failure Chf Treatment Devices market in 2033?
Congestive Heart Failure Chf Treatment Devices Industry Analysis
Congestive Heart Failure Chf Treatment Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Congestive Heart Failure Chf Treatment Devices Market Analysis Report by Region
Europe Congestive Heart Failure Chf Treatment Devices Market Report:
Europe's CHF treatment devices market is projected to grow from $2.96 billion in 2023 to $4.88 billion by 2033. Aging demographics and increased adoption of sophisticated medical devices are key growth drivers, complemented by supportive regulations for device innovation.Asia Pacific Congestive Heart Failure Chf Treatment Devices Market Report:
In the Asia Pacific region, the market is expected to grow from $1.88 billion in 2023 to $3.10 billion by 2033, reflecting the region's increasing healthcare spending and rising incidences of heart disease. Additionally, enhanced patient awareness and improved access to healthcare services will drive market penetration.North America Congestive Heart Failure Chf Treatment Devices Market Report:
North America remains the largest market, expected to grow from $3.69 billion in 2023 to $6.07 billion by 2033. This growth is underpinned by advanced healthcare infrastructure, significant investment in R&D, and a high prevalence of CHF, alongside aggressive marketing by major players.South America Congestive Heart Failure Chf Treatment Devices Market Report:
The South American market for CHF treatment devices is comparatively smaller, projected to expand from $0.21 billion in 2023 to $0.35 billion by 2033. The growth is driven by the burgeoning middle-class population, but challenges such as economic instability may hinder progress.Middle East & Africa Congestive Heart Failure Chf Treatment Devices Market Report:
The Middle East and Africa market is estimated to rise from $1.25 billion in 2023 to $2.06 billion by 2033. Growth in this region will be facilitated by rising healthcare investments and greater access to advanced medical technologies.Tell us your focus area and get a customized research report.
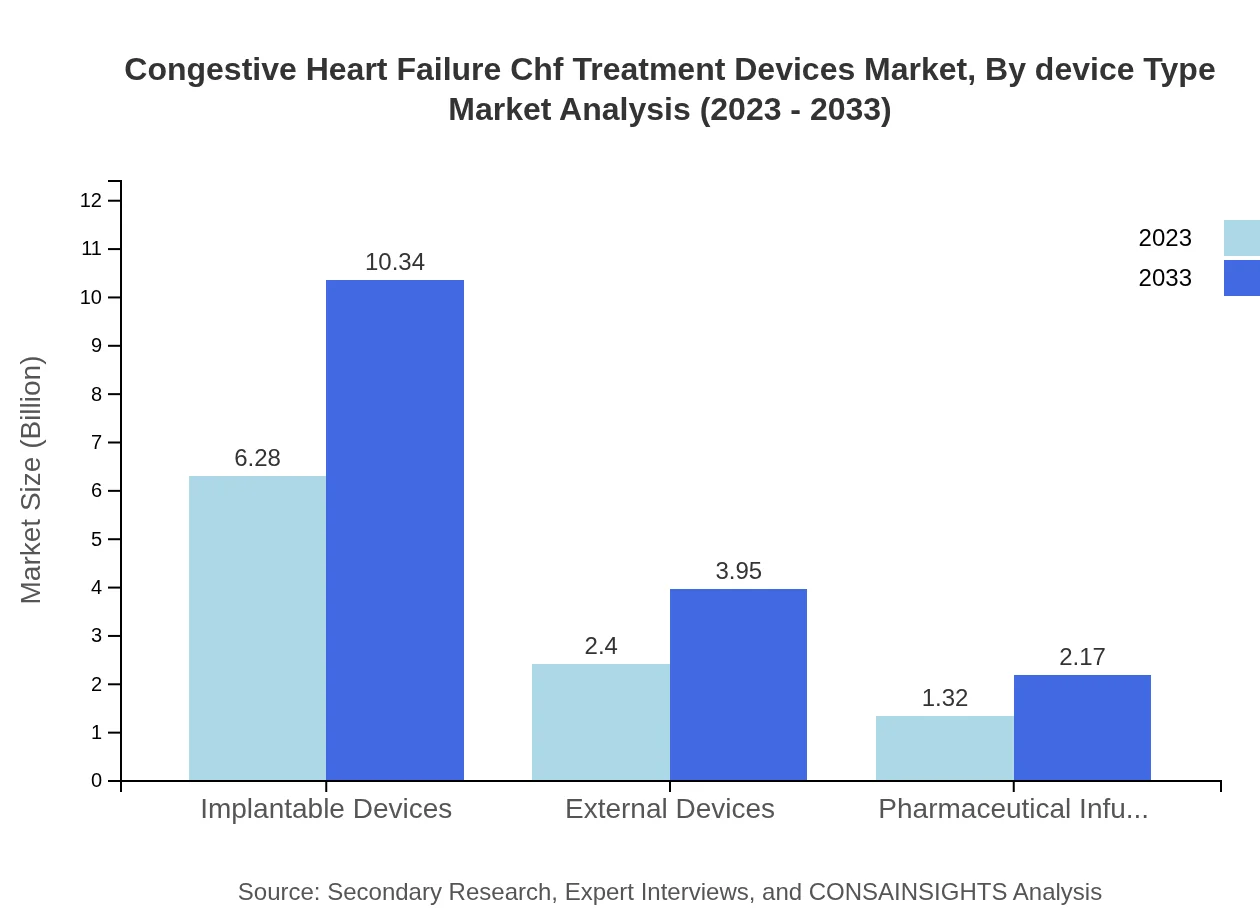
Congestive Heart Failure Chf Treatment Devices Market Analysis By Device Type
The market for CHF treatment devices can be segmented into implantable devices and external devices. Implantable devices, such as cardiac resynchronization therapy devices, are anticipated to hold a significant share due to their effectiveness. External devices, including wearable monitors, are gaining traction in managing patient health remotely.
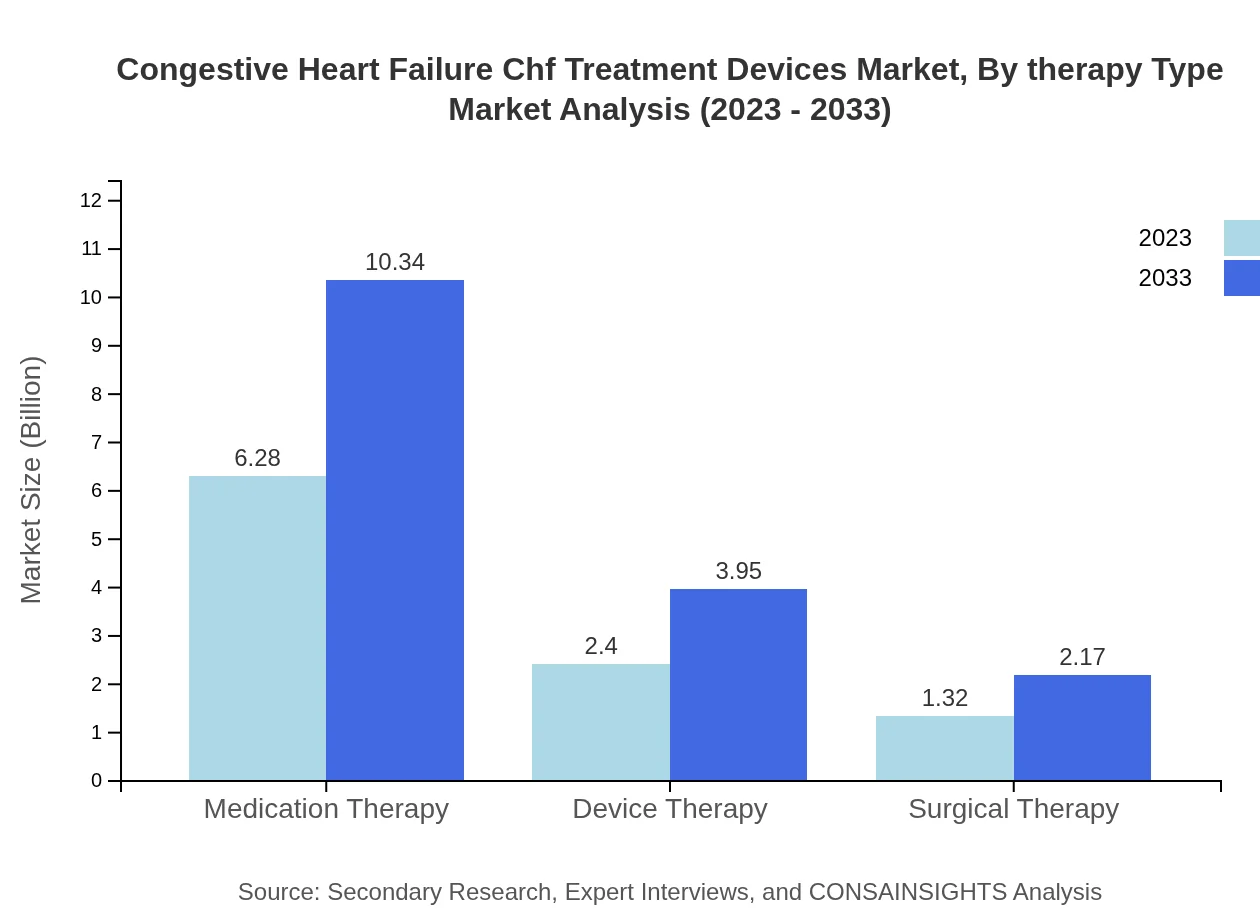
Congestive Heart Failure Chf Treatment Devices Market Analysis By Therapy Type
Therapy types include medication therapy, device therapy, and surgical therapy. Medication therapy continues to dominate the market due to its wide usage, but device therapy is gaining momentum as technological advancements enhance treatment efficacy and patient monitoring capabilities.
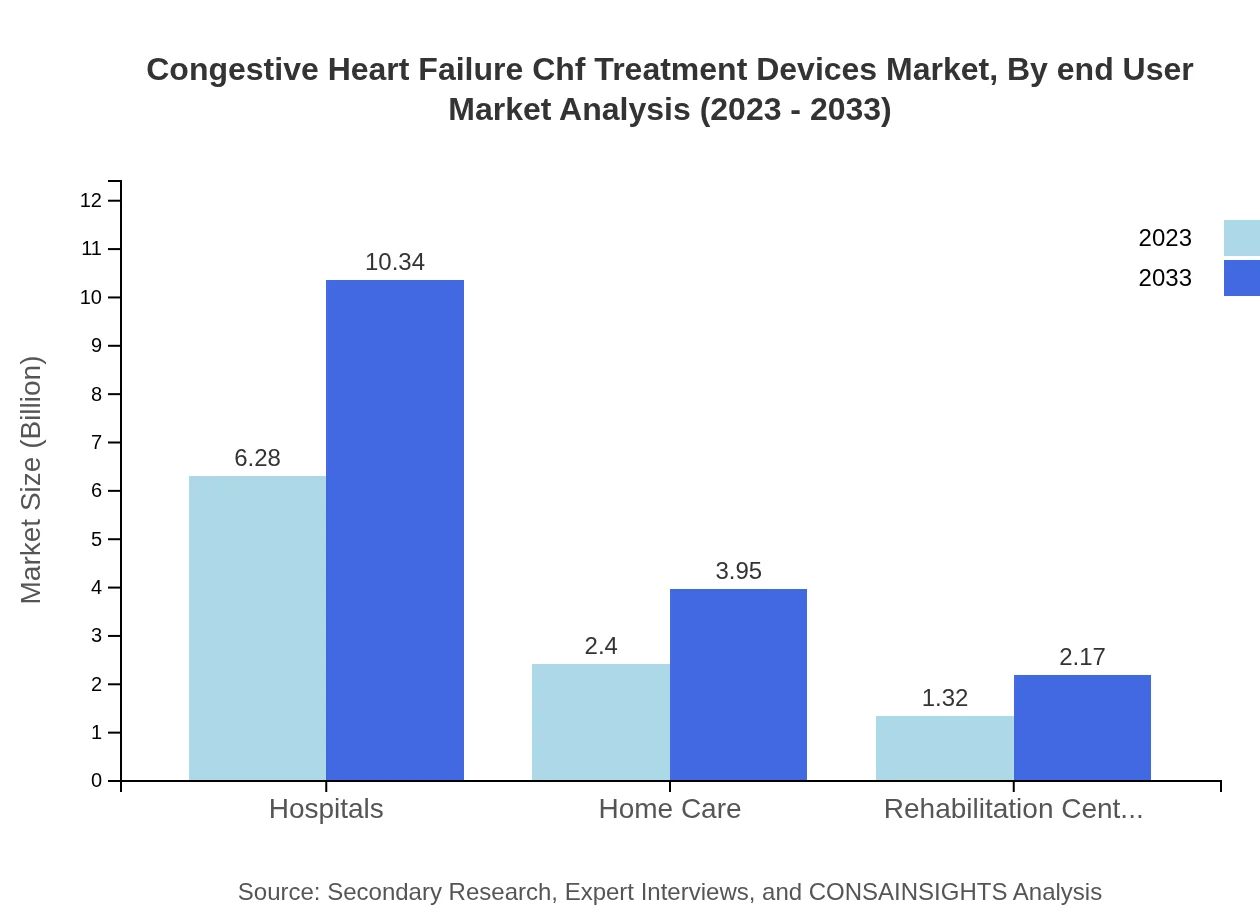
Congestive Heart Failure Chf Treatment Devices Market Analysis By End User
The primary end-users include hospitals, home care settings, and rehabilitation centers. Hospitals constitute the largest share of the market, driven by acute care settings, while home care is emerging as a critical segment due to the increasing trend of decentralized healthcare delivery.
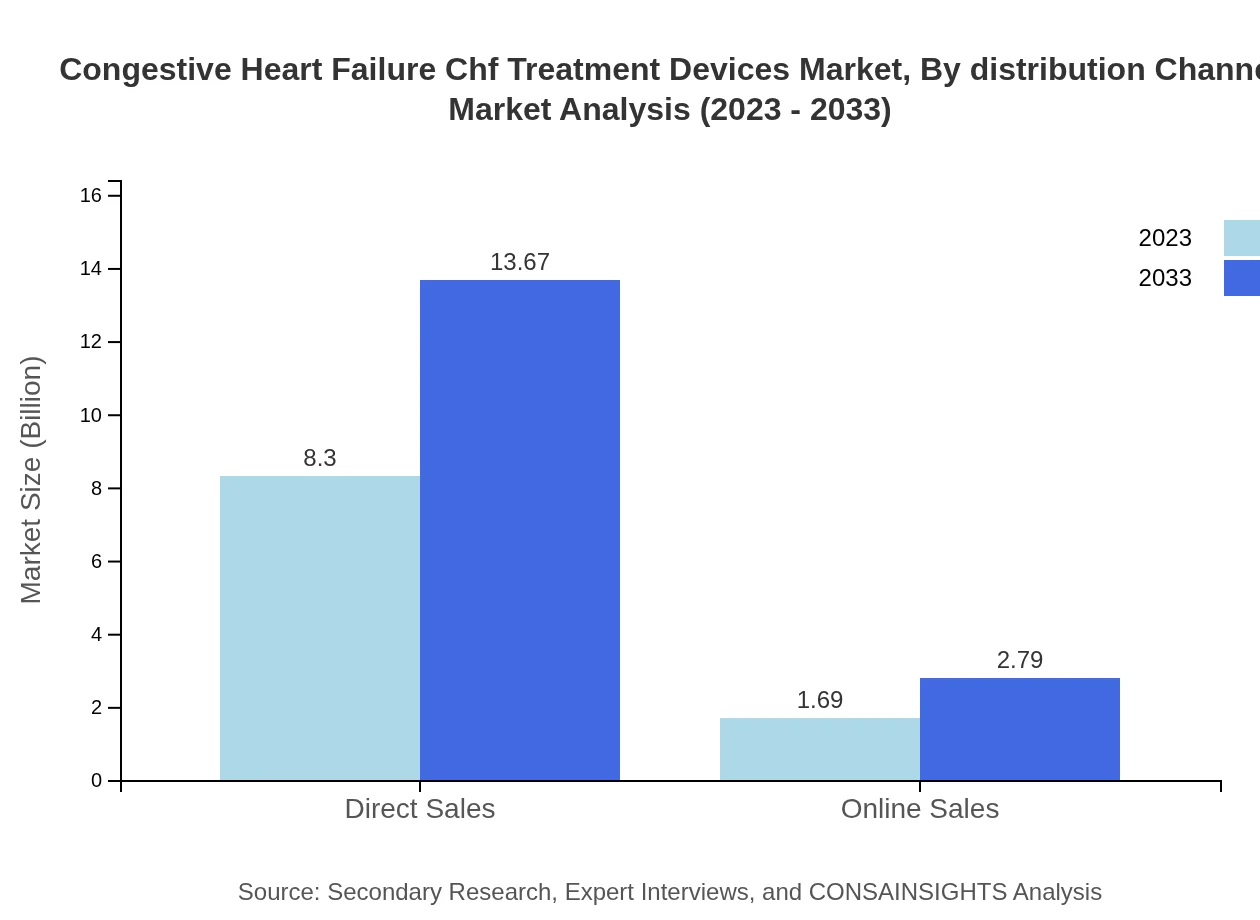
Congestive Heart Failure Chf Treatment Devices Market Analysis By Distribution Channel
Distribution channels include direct sales and online sales. Direct sales dominate the market for CHF treatment devices, attributed to strong relationships between manufacturers and healthcare providers. Online sales are also growing as patients become more comfortable with digital health solutions.
Congestive Heart Failure Chf Treatment Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Congestive Heart Failure Chf Treatment Devices Industry
Medtronic :
A global leader in medical technology, Medtronic focuses on developing breakthrough technologies for heart disease management, particularly in implanted devices.Abbott Laboratories:
Abbott is renowned for its innovative cardiovascular devices, including heart pumps and cardiac monitoring systems, playing a crucial role in CHF management.Boston Scientific:
Boston Scientific specializes in developing less invasive options for treating heart conditions, including implantable devices that improve patient outcomes.Biotronik:
Biotronik provides comprehensive cardiovascular solutions focusing on personalized therapy and patient monitoring for CHF patients.Philips Healthcare:
Philips contributes to CHF management with its advanced monitoring technologies and integrated healthcare solutions aimed at enhancing patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of congestive Heart Failure Chf Treatment Devices?
The global market size for Congestive Heart Failure (CHF) treatment devices is projected at $10 billion in 2023, with an expected growth to reach approximately $16.3 billion by 2033, reflecting a CAGR of 5% over the forecast period.
What are the key market players or companies in this congestive Heart Failure Chf Treatment Devices industry?
Key players in the CHF treatment device industry include major medical device manufacturers and companies specializing in heart failure management, contributing to innovation and competitive strategies to enhance market growth and patient outcomes.
What are the primary factors driving the growth in the congestive Heart Failure Chf Treatment Devices industry?
Growth in the CHF treatment devices market is primarily driven by the increasing prevalence of heart failure, advancements in medical technology, rising geriatric population, and heightened awareness and diagnosis of heart-related conditions.
Which region is the fastest Growing in the congestive Heart Failure Chf Treatment Devices?
The Asia Pacific region is projected to be the fastest-growing market for CHF treatment devices, expanding from $1.88 billion in 2023 to $3.10 billion by 2033, as healthcare infrastructure and access to advanced treatments improve.
Does ConsaInsights provide customized market report data for the congestive Heart Failure Chf Treatment Devices industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the CHF treatment devices industry, ensuring relevant insights and data to support strategic decision-making.
What deliverables can I expect from this congestive Heart Failure Chf Treatment Devices market research project?
Deliverables from the CHF market research project typically include comprehensive market analysis reports, segment-wise evaluations, competitive landscape assessments, and forecasts on growth trends and regional performance.
What are the market trends of congestive Heart Failure Chf Treatment Devices?
Current trends in the CHF treatment devices market include increasing adoption of telehealth technologies, growth in home care settings, a shift toward minimally invasive procedures, and heightened focus on personalized medicine.