Conjugate Vaccine Market Report
Published Date: 31 January 2026 | Report Code: conjugate-vaccine
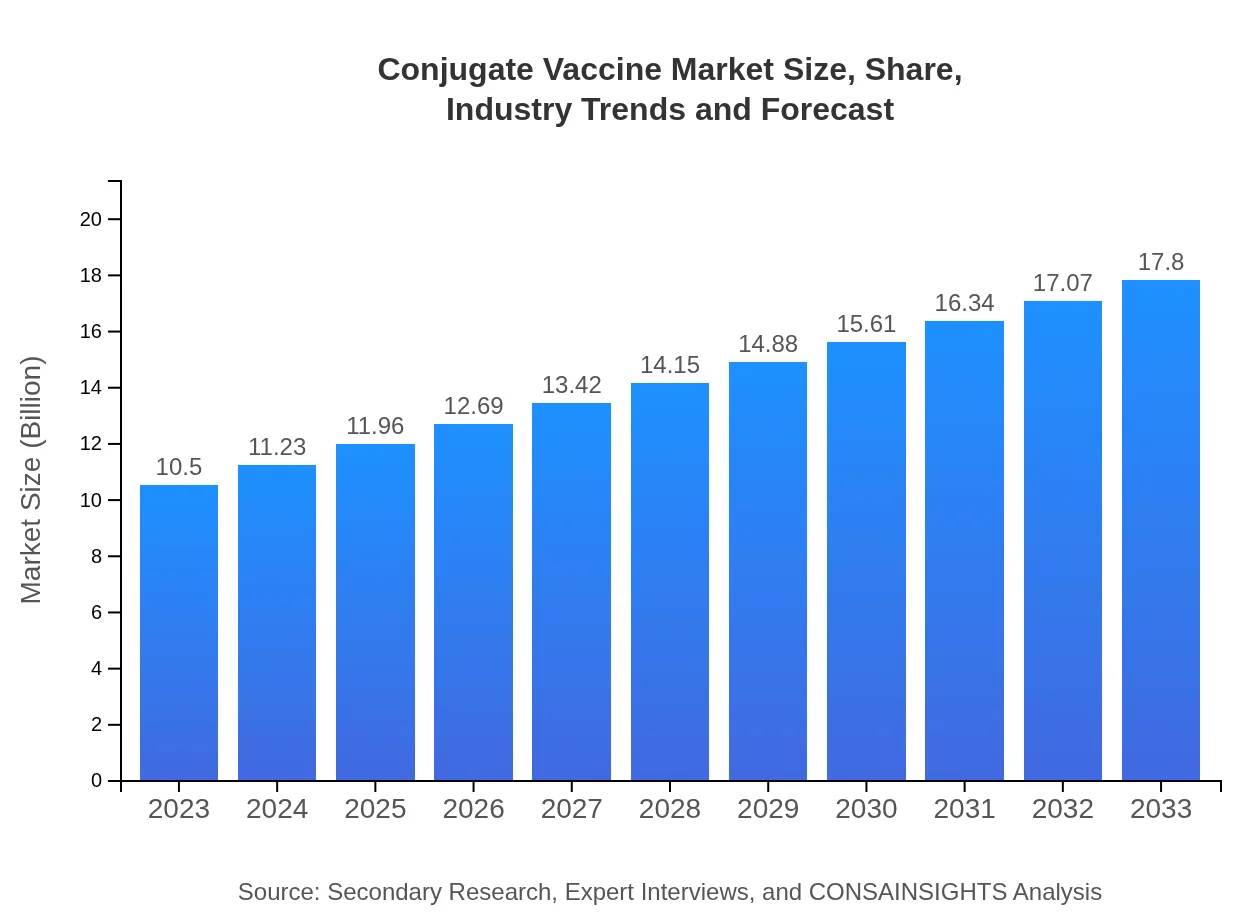
Conjugate Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the global conjugate vaccine market, covering market size, trends, and forecasts for 2023-2033. It offers insights into regional dynamics, segmentation, and emerging technologies that are shaping the future of this critical sector in immunization.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 5.3% |
| 2033 Market Size | $17.80 Billion |
| Top Companies | Pfizer Inc., Sanofi Pasteur, GlaxoSmithKline, Merck & Co., Inc., Baxter International Inc. |
| Last Modified Date | 31 January 2026 |
Conjugate Vaccine Market Overview
Customize Conjugate Vaccine Market Report market research report
- ✔ Get in-depth analysis of Conjugate Vaccine market size, growth, and forecasts.
- ✔ Understand Conjugate Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Conjugate Vaccine
What is the Market Size & CAGR of the Conjugate Vaccine market in 2023?
Conjugate Vaccine Industry Analysis
Conjugate Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Conjugate Vaccine Market Analysis Report by Region
Europe Conjugate Vaccine Market Report:
Europe's conjugate vaccine market is expected to rise from $3.56 billion in 2023 to $6.03 billion by 2033, driven by supportive regulatory frameworks and increased public health funding to combat infectious diseases.Asia Pacific Conjugate Vaccine Market Report:
The Asia Pacific region is poised for substantial growth, with the market expected to increase from $1.75 billion in 2023 to $2.96 billion by 2033. The rise is attributed to increasing healthcare expenditure and expanded immunization programs addressing both communicable and non-communicable diseases.North America Conjugate Vaccine Market Report:
North America is projected to see growth from $3.71 billion in 2023 to $6.29 billion by 2033. The region benefits from advanced healthcare infrastructure, awareness campaigns, and research initiatives focused on innovative vaccine development.South America Conjugate Vaccine Market Report:
In South America, the conjugate vaccine market is forecasted to grow from $0.15 billion in 2023 to $0.26 billion by 2033. This growth is influenced by improved public health policies and efforts to enhance vaccination coverage in underserved communities.Middle East & Africa Conjugate Vaccine Market Report:
The market in the Middle East and Africa is anticipated to grow from $1.33 billion in 2023 to $2.26 billion by 2033, spurred by international health partnerships and programs aimed at improving immunization rates and accessibility.Tell us your focus area and get a customized research report.
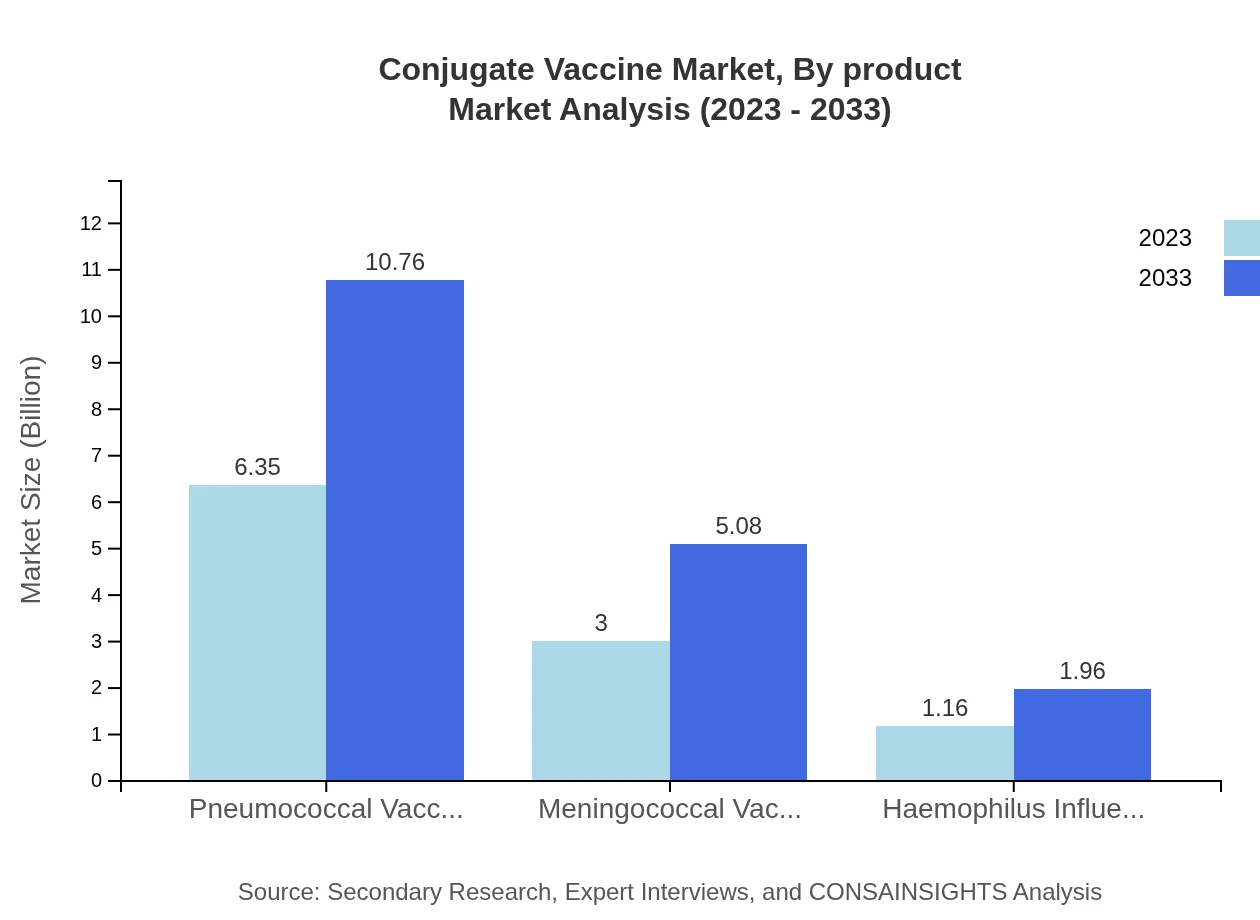
Conjugate Vaccine Market Analysis By Product
In the conjugate vaccine market, pneumococcal vaccines dominate with a size of $6.35 billion in 2023 and projected growth to $10.76 billion by 2033, accounting for 60.44% market share throughout the period. Meningococcal and Haemophilus influenzae type b vaccines also show notable growth, with meningococcal vaccines expected to rise from $3.00 billion to $5.08 billion and Hib vaccines from $1.16 billion to $1.96 billion.
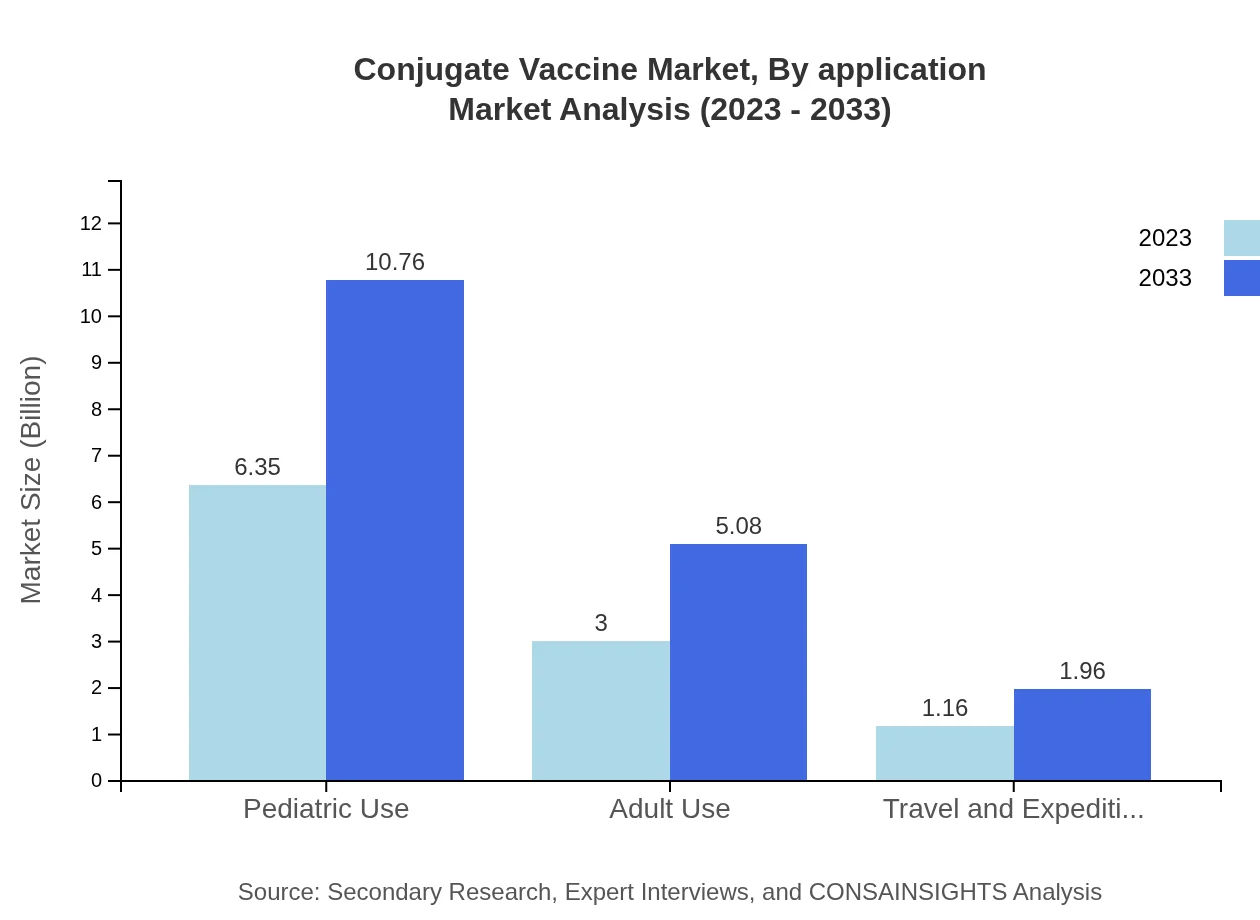
Conjugate Vaccine Market Analysis By Application
The conjugate vaccine market is significantly utilized in pediatric applications, projected to increase from $6.35 billion in 2023 to $10.76 billion by 2033, dominating the market share at 60.44%. Adult and travel expedition applications are also growing; adult use is expected to rise from $3.00 billion to $5.08 billion, while travel vaccinations grow from $1.16 billion to $1.96 billion.
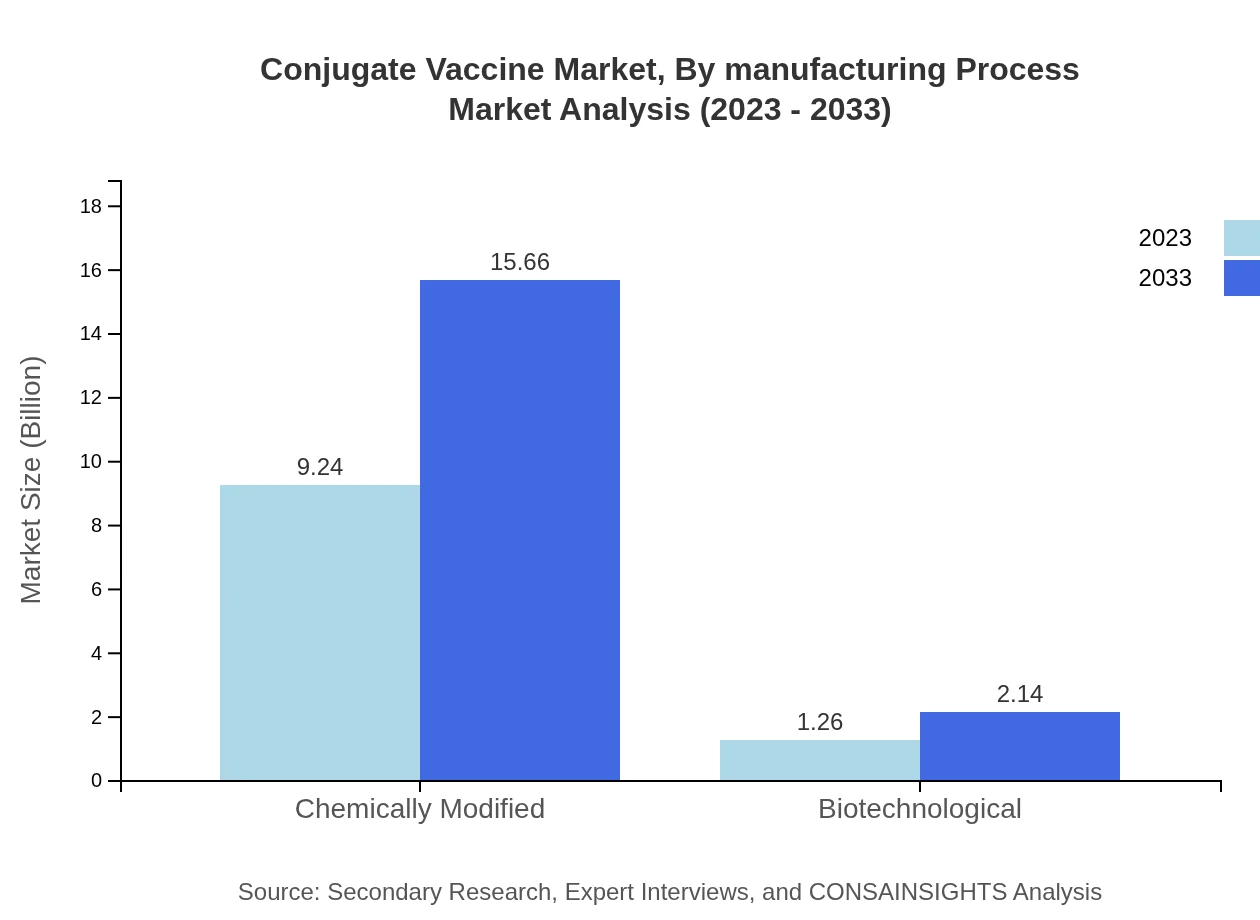
Conjugate Vaccine Market Analysis By Manufacturing Process
The market is heavily skewed towards chemically modified vaccines, projected to grow from $9.24 billion in 2023 to $15.66 billion by 2033, representing 87.96% market share. Biotechnologically produced vaccines are expected to rise from $1.26 billion to $2.14 billion, albeit at a smaller market share.
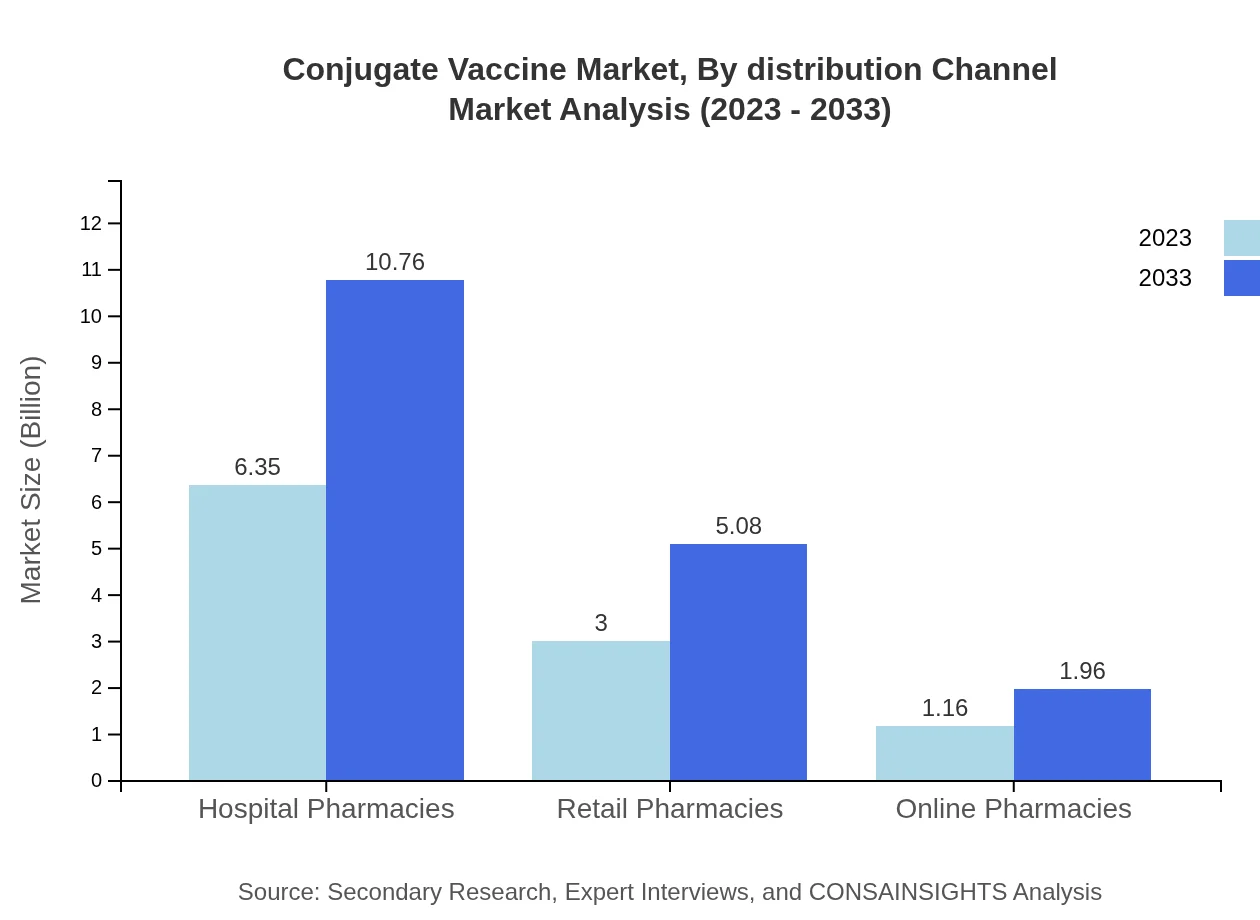
Conjugate Vaccine Market Analysis By Distribution Channel
Hospital pharmacies lead in distribution, with a size of $6.35 billion in 2023, growing to $10.76 billion by 2033, while retail pharmacies anticipate growth from $3.00 billion to $5.08 billion. Online pharmacies, though smaller, are expected to rise from $1.16 billion to $1.96 billion, reflecting changing consumer purchasing behaviors.
Conjugate Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Conjugate Vaccine Industry
Pfizer Inc.:
A leader in global pharmaceuticals, Pfizer specializes in vaccine development and distribution, known for its pneumococcal conjugate vaccine.Sanofi Pasteur:
One of the largest vaccine manufacturers globally, Sanofi Pasteur develops a range of conjugate vaccines, focusing on innovation and public health improvements.GlaxoSmithKline:
GSK plays a significant role in the conjugate vaccine sector, particularly with its meningococcal and Hib vaccines.Merck & Co., Inc.:
A prominent figure in vaccine research, Merck is involved in producing vaccines including those targeting bacterial infections.Baxter International Inc.:
Baxter focuses on biopharmaceuticals and vaccine production, ensuring wide access to conjugate vaccines worldwide.We're grateful to work with incredible clients.









FAQs
What is the market size of conjugate Vaccine?
The global conjugate vaccine market is valued at approximately $10.5 billion in 2023, with an expected compound annual growth rate (CAGR) of 5.3% over the next decade, projecting significant growth and demand in the healthcare landscape.
What are the key market players or companies in this conjugate vaccine industry?
Key players in the conjugate vaccine market include Pfizer, Merck, GlaxoSmithKline, and Sanofi Pasteur, which are leading the way in innovative product development and clinical research, thus establishing a competitive edge in the immunization landscape.
What are the primary factors driving the growth in the conjugate vaccine industry?
The growth in the conjugate vaccine industry is primarily driven by increased vaccine awareness, rising incidences of vaccine-preventable diseases, advancements in vaccine technology, and supportive government initiatives promoting immunization programs globally.
Which region is the fastest Growing in the conjugate vaccine?
The fastest-growing region in the conjugate vaccine market is Europe, with a market size projected to increase from $3.56 billion in 2023 to $6.03 billion by 2033, highlighting strong market potential and healthcare investments in immunization.
Does ConsaInsights provide customized market report data for the conjugate vaccine industry?
Yes, ConsaInsights offers customized market report data tailored to client-specific needs within the conjugate vaccine industry, ensuring insightful, actionable intelligence to aid strategic decision-making and market positioning.
What deliverables can I expect from this conjugate vaccine market research project?
Deliverables from the conjugate vaccine market research project include comprehensive market analysis, competitive landscape assessment, revenue and segment forecasts, and actionable insights supported by qualitative and quantitative data.
What are the market trends of conjugate vaccine?
Current trends in the conjugate vaccine market include a shift towards pediatric vaccinations, increased research in biosimilar vaccines, and the expansion of immunization coverage in emerging markets, aligning with global health priorities.