Coronary Artery Disease Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: coronary-artery-disease-therapeutics
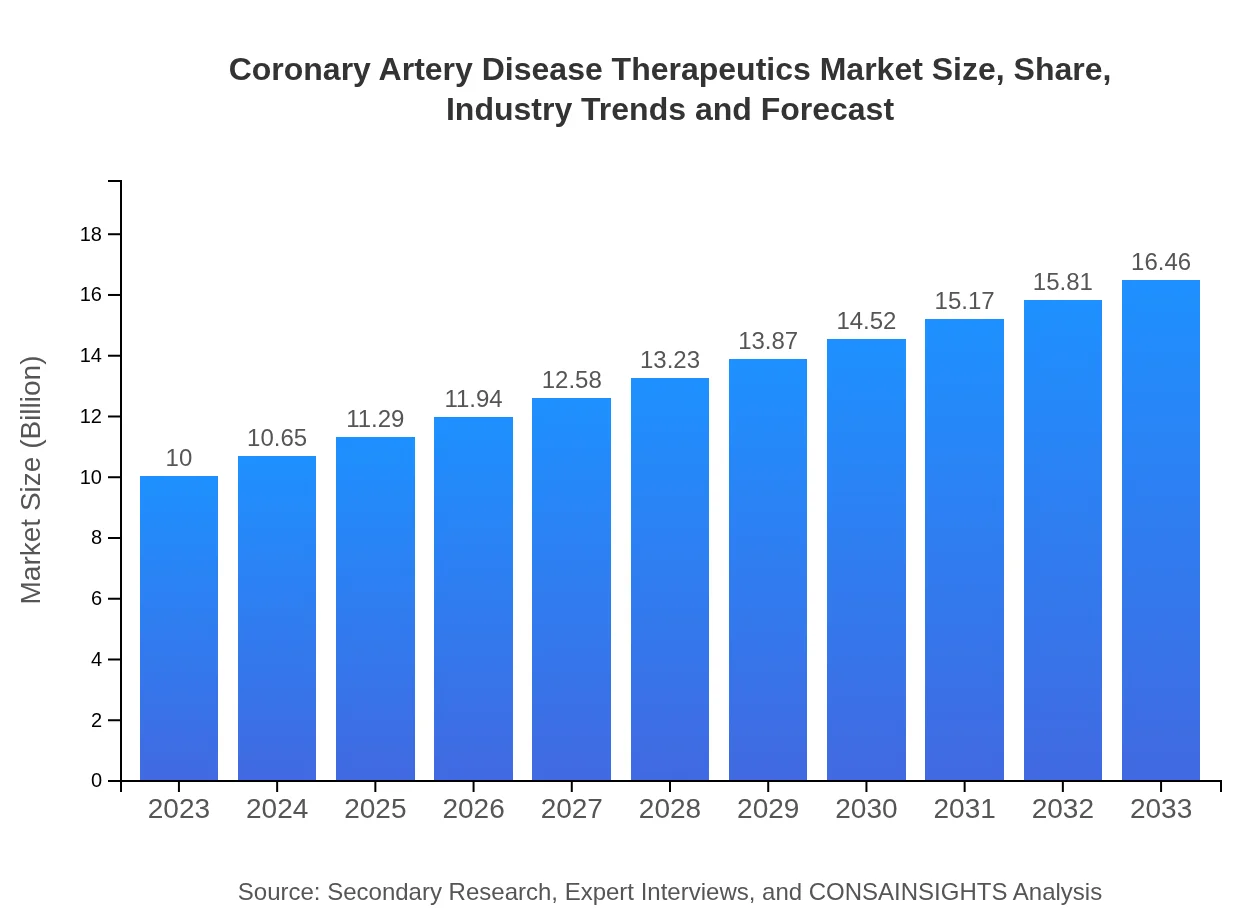
Coronary Artery Disease Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Coronary Artery Disease Therapeutics market, detailing various insights including market size, anticipated growth trends, and regional breakdowns from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | Pfizer Inc., Bayer AG, Bristol-Myers Squibb, AstraZeneca |
| Last Modified Date | 31 January 2026 |
Coronary Artery Disease Therapeutics Market Overview
Customize Coronary Artery Disease Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Coronary Artery Disease Therapeutics market size, growth, and forecasts.
- ✔ Understand Coronary Artery Disease Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Coronary Artery Disease Therapeutics
What is the Market Size & CAGR of Coronary Artery Disease Therapeutics market in {Year}?
Coronary Artery Disease Therapeutics Industry Analysis
Coronary Artery Disease Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Coronary Artery Disease Therapeutics Market Analysis Report by Region
Europe Coronary Artery Disease Therapeutics Market Report:
Europe's market size was $2.66 billion in 2023, projected to grow to $4.38 billion by 2033. Strict regulatory frameworks and early adoption of advanced therapy options enhance market growth across the continent.Asia Pacific Coronary Artery Disease Therapeutics Market Report:
In the Asia-Pacific region, the market size was $2.19 billion in 2023 and is projected to reach $3.60 billion by 2033, exhibiting robust growth due to increasing urbanization and lifestyle changes contributing to cardiovascular diseases.North America Coronary Artery Disease Therapeutics Market Report:
North America dominates the CAD therapeutics market, with a size of $3.39 billion in 2023 estimated to rise to $5.58 billion by 2033. This can be attributed to advanced healthcare facilities and increasing awareness of cardiovascular health.South America Coronary Artery Disease Therapeutics Market Report:
The South American market for Coronary Artery Disease Therapeutics stood at $0.66 billion in 2023 and is expected to expand to $1.09 billion by 2033. Government initiatives aimed at improving health infrastructure are significant growth drivers.Middle East & Africa Coronary Artery Disease Therapeutics Market Report:
In the Middle East and Africa, the market is expected to increase from $1.10 billion in 2023 to $1.81 billion by 2033, driven by the rising healthcare expenditure and increasing prevalence of lifestyle diseases.Tell us your focus area and get a customized research report.
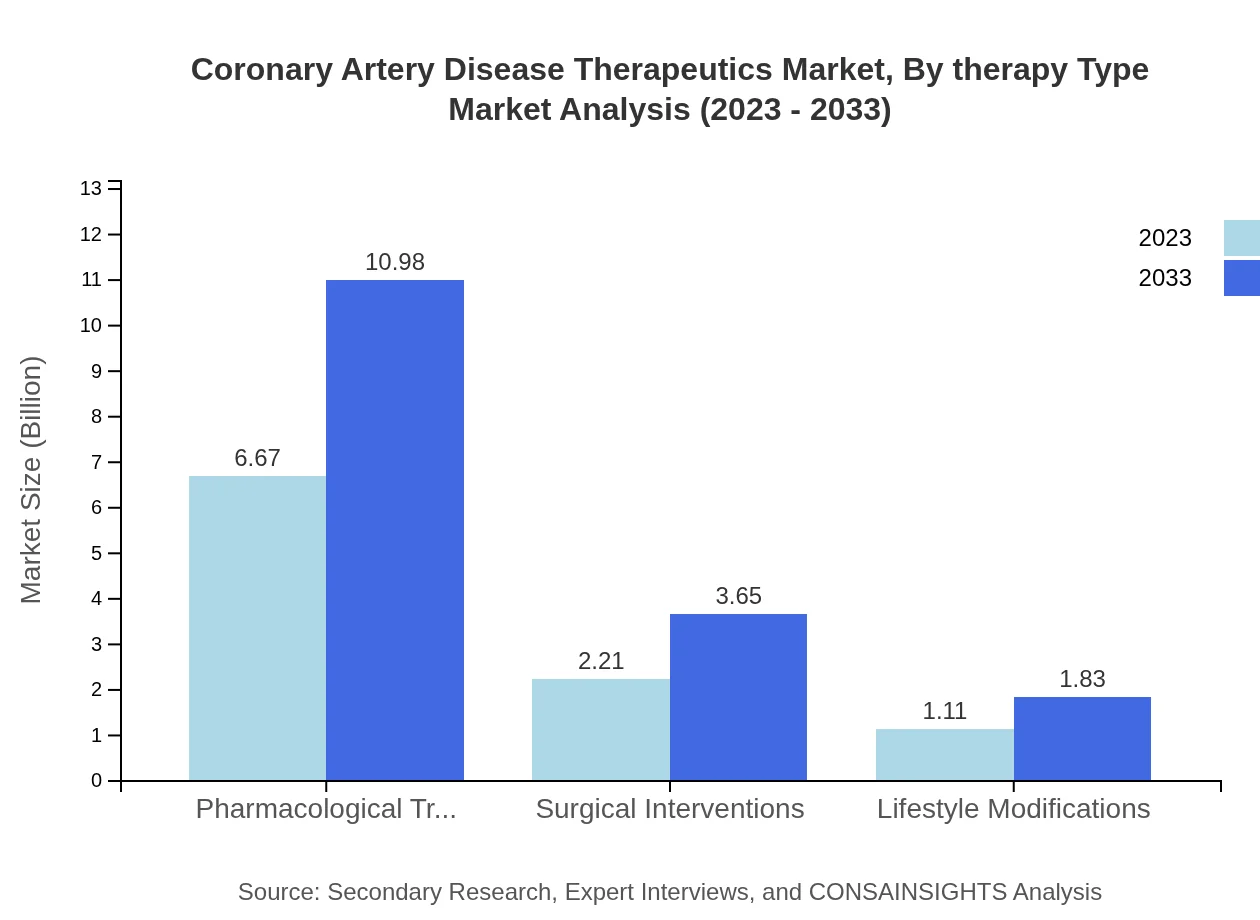
Coronary Artery Disease Therapeutics Market Analysis By Therapy Type
The market for Coronary Artery Disease treatments by therapy type is dominated by pharmacological treatments, including antiplatelet drugs and beta-blockers, which together comprise a significant market share, forecasted at $6.67 billion for antiplatelet drugs and $2.21 billion for beta-blockers in 2023.
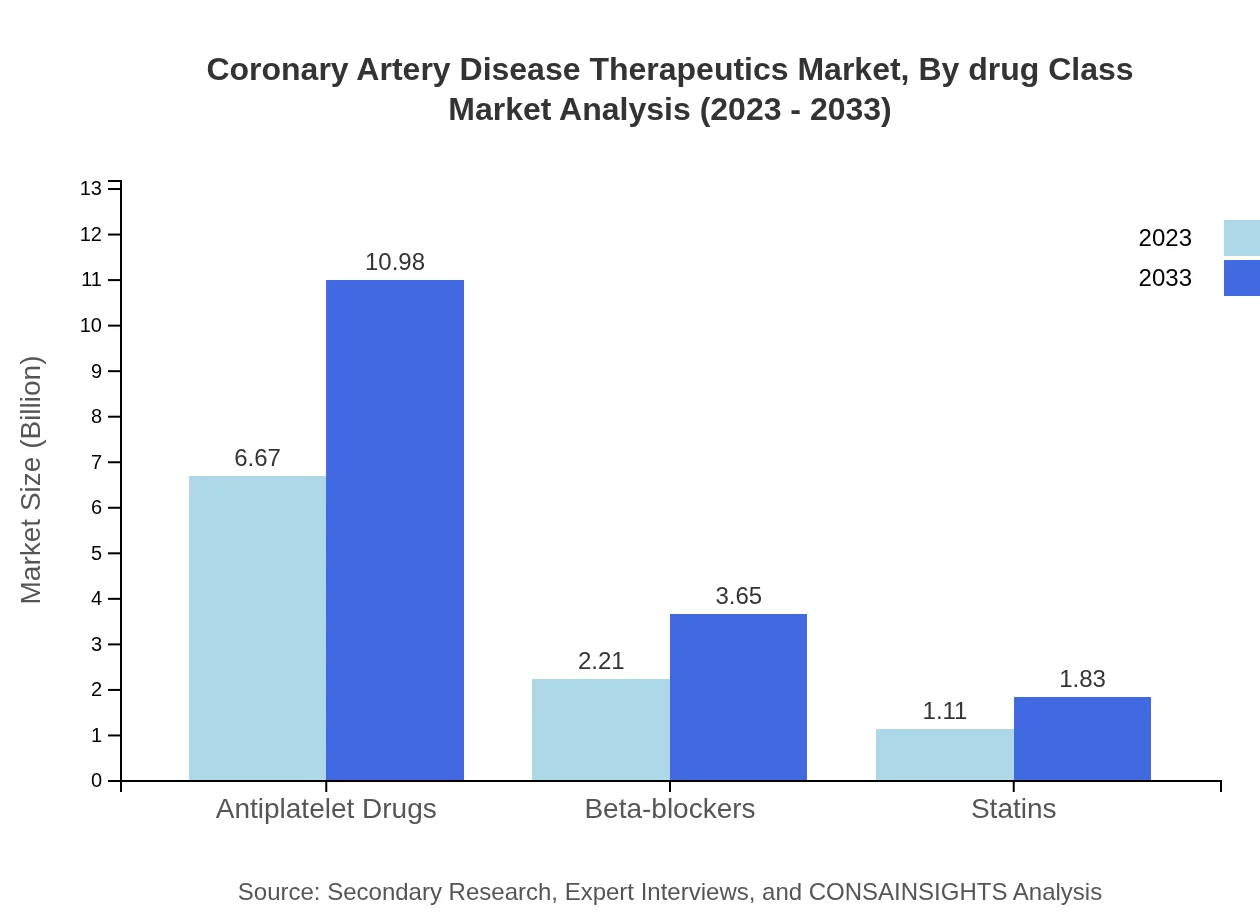
Coronary Artery Disease Therapeutics Market Analysis By Drug Class
In terms of drug class, antiplatelet agents hold a substantial market share, accounting for approximately 66.73% of the market in 2023. Statins follow at 11.12%, highlighting their importance in cholesterol management among CAD patients.
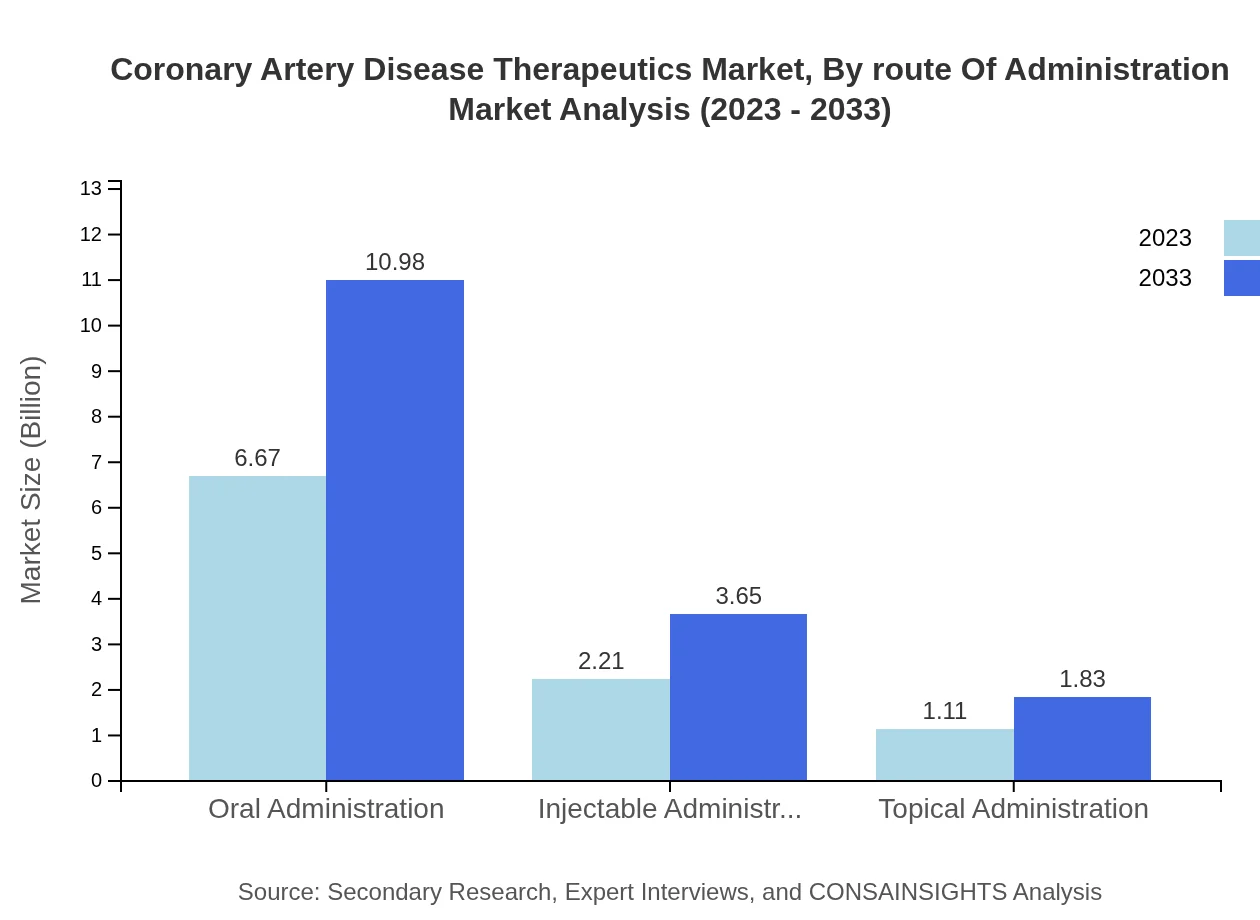
Coronary Artery Disease Therapeutics Market Analysis By Route Of Administration
Oral administration remains the preferred route, comprising 66.73% of the market share in 2023, leveraging patient compliance and convenience. Injectable administration captures 22.15%, showcasing its significance for acute therapeutic interventions.
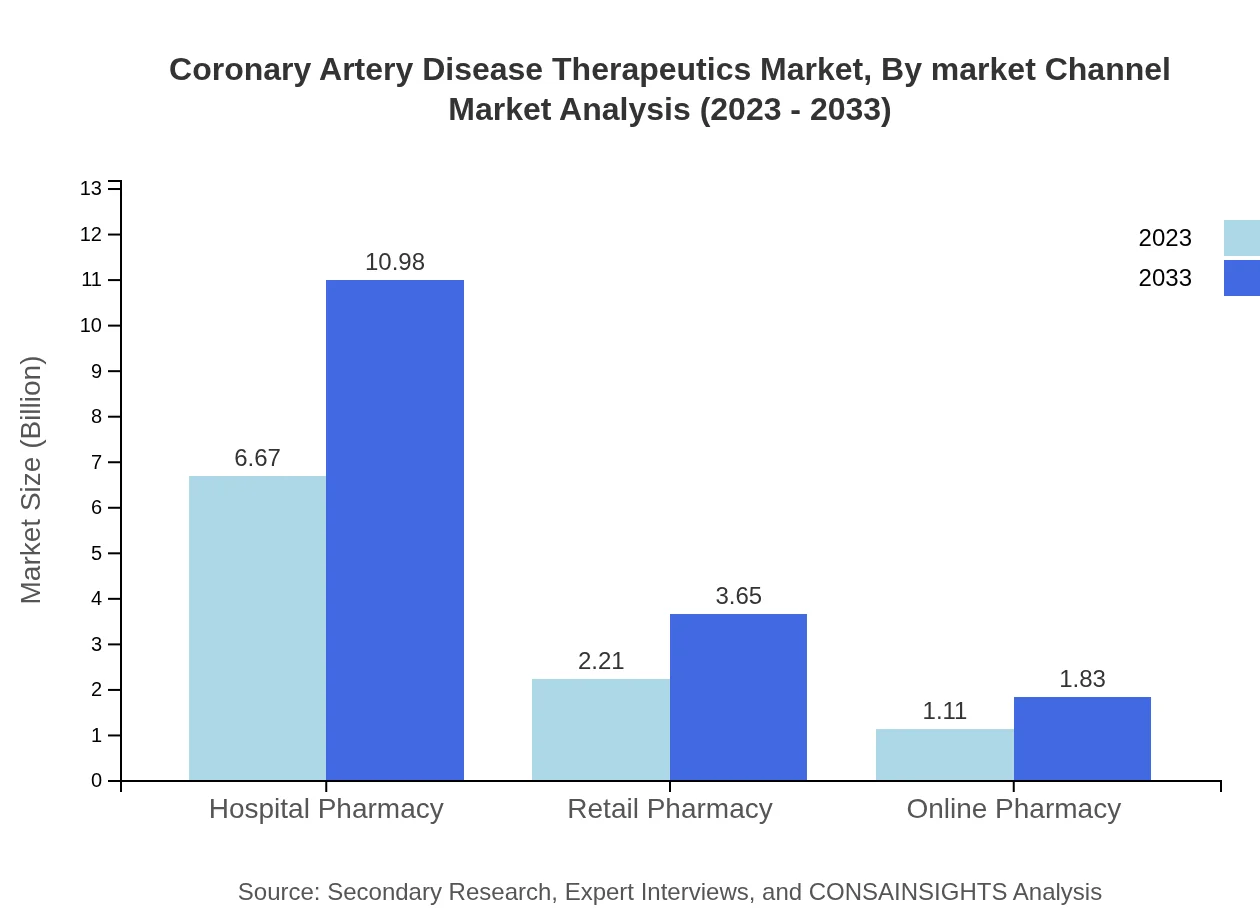
Coronary Artery Disease Therapeutics Market Analysis By Market Channel
Hospital pharmacies dominate the CAD therapeutics market at 66.73%, providing critical access to a range of therapies, while retail pharmacies and online channels account for 22.15% and 11.12% respectively, reflecting consumer trends toward convenience.
Coronary Artery Disease Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Coronary Artery Disease Therapeutics Industry
Pfizer Inc.:
A leading global biopharmaceutical company known for its innovative pharmacological therapies including antiplatelet drugs.Bayer AG:
Renowned for its research and development in cardiovascular drugs, offering comprehensive solutions for CAD patients.Bristol-Myers Squibb:
Specializes in cardiovascular medicine, contributing significantly with new drug discoveries and established therapies.AstraZeneca:
A key player in CAD therapeutics, focusing on statin therapies and advanced drug delivery systems.We're grateful to work with incredible clients.









FAQs
What is the market size of coronary artery disease therapeutics?
The coronary artery disease therapeutics market is valued at approximately $10 billion in 2023 and is projected to grow at a CAGR of 5% over the next decade, reaching significant expansion by 2033.
What are the key market players or companies in the coronary artery disease therapeutics industry?
Key players in the coronary artery disease therapeutics market include multinational pharmaceutical companies and specialized biotech firms focusing on innovative treatment methods and drug development strategies to enhance patient care.
What are the primary factors driving the growth in the coronary artery disease therapeutics industry?
The growth of the coronary artery disease therapeutics industry is propelled by rising prevalence rates, advancements in medical technologies, an aging population, and increased awareness regarding cardiovascular health.
Which region is the fastest Growing in the coronary artery disease therapeutics?
North America is the fastest-growing region, with a market size projected to expand from $3.39 billion in 2023 to $5.58 billion by 2033, driven by advanced healthcare infrastructure and high demand for effective therapies.
Does ConsaInsights provide customized market report data for the coronary artery disease therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients interested in detailed insights and strategic analysis of the coronary artery disease therapeutics industry.
What deliverables can I expect from this coronary artery disease therapeutics market research project?
Expect comprehensive deliverables including detailed market analysis, forecasts, trends, and segmentation data, ensuring insightful knowledge to guide strategic decisions in the coronary artery disease therapeutics market.
What are the market trends of coronary artery disease therapeutics?
Current trends in the coronary artery disease therapeutics market emphasize a shift towards personalized medicine, innovative drug delivery systems, and a growing focus on preventive care and lifestyle modifications in treatment plans.