Coronary Stents Market Report
Published Date: 31 January 2026 | Report Code: coronary-stents
Coronary Stents Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Coronary Stents market, highlighting key trends, forecasts, and insights from 2023 to 2033. It examines market segmentation, regional dynamics, and technological advancements, presenting a comprehensive view of the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
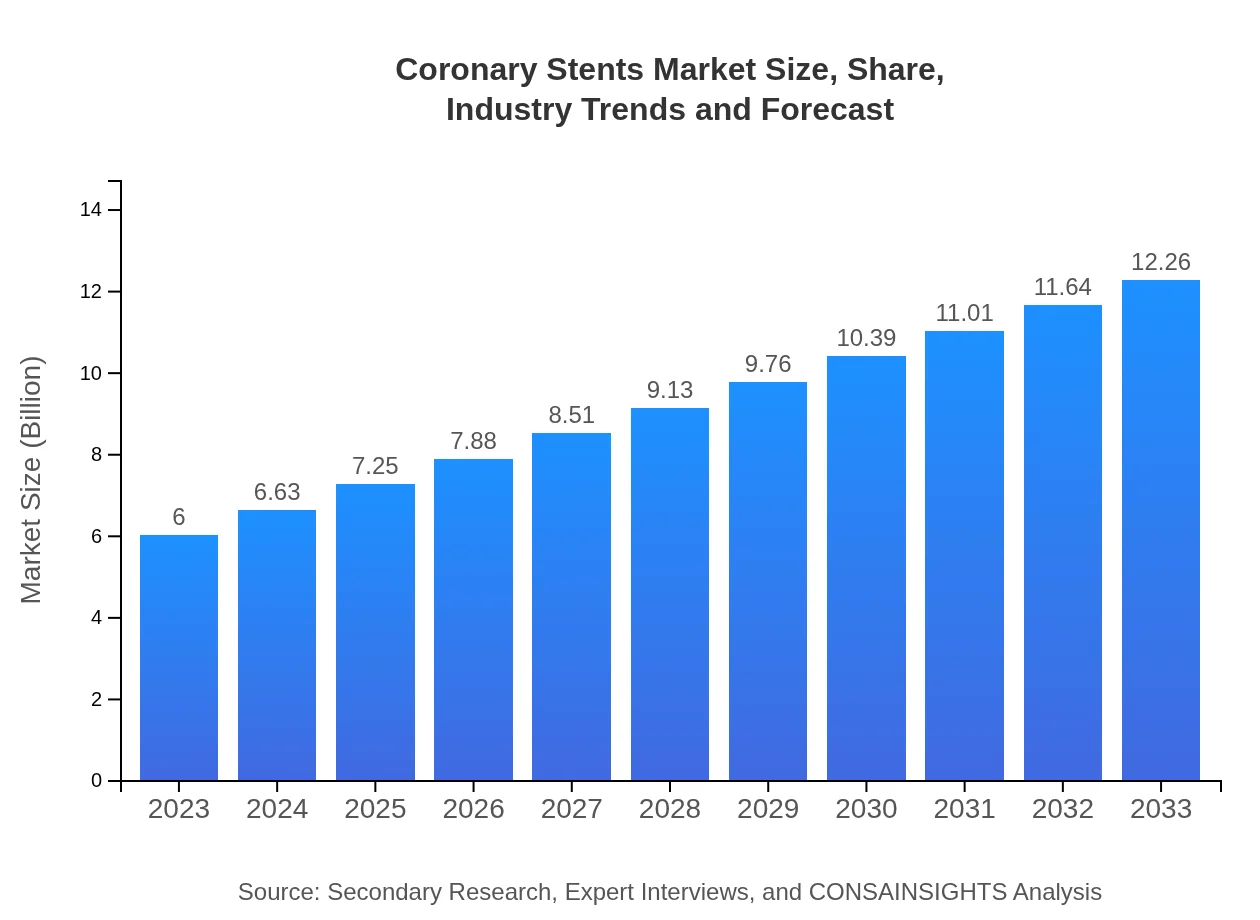
| 2023 Market Size | $6.00 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $12.26 Billion |
| Top Companies | Medtronic , Abbott Laboratories, Boston Scientific, B.Braun, Terumo Corporation |
| Last Modified Date | 31 January 2026 |
Coronary Stents Market Overview
Customize Coronary Stents Market Report market research report
- ✔ Get in-depth analysis of Coronary Stents market size, growth, and forecasts.
- ✔ Understand Coronary Stents's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Coronary Stents
What is the Market Size & CAGR of Coronary Stents market in 2023?
Coronary Stents Industry Analysis
Coronary Stents Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Coronary Stents Market Analysis Report by Region
Europe Coronary Stents Market Report:
The European market for Coronary Stents is forecasted to expand from $1.64 billion in 2023 to $3.35 billion by 2033, driven by innovation in stent manufacturing and a robust regulatory framework supporting new product launches.Asia Pacific Coronary Stents Market Report:
The Coronary Stents market in the Asia Pacific region is projected to grow from $1.17 billion in 2023 to $2.40 billion by 2033, driven by increasing healthcare investments and rising incidences of cardiovascular diseases.North America Coronary Stents Market Report:
North America holds a significant share of the market, projected to grow from $1.97 billion in 2023 to $4.02 billion by 2033, attributed to high healthcare expenditure, advanced healthcare infrastructure, and the growing demand for interventional cardiology.South America Coronary Stents Market Report:
In South America, the market is expected to rise from $0.48 billion in 2023 to $0.99 billion by 2033, with healthcare improvements and an expanding middle-class population increasing access to advanced medical technologies.Middle East & Africa Coronary Stents Market Report:
In the Middle East and Africa, the market is estimated to grow from $0.74 billion in 2023 to $1.50 billion by 2033, supported by increasing healthcare investments and awareness about heart diseases.Tell us your focus area and get a customized research report.
Coronary Stents Market Analysis By Product
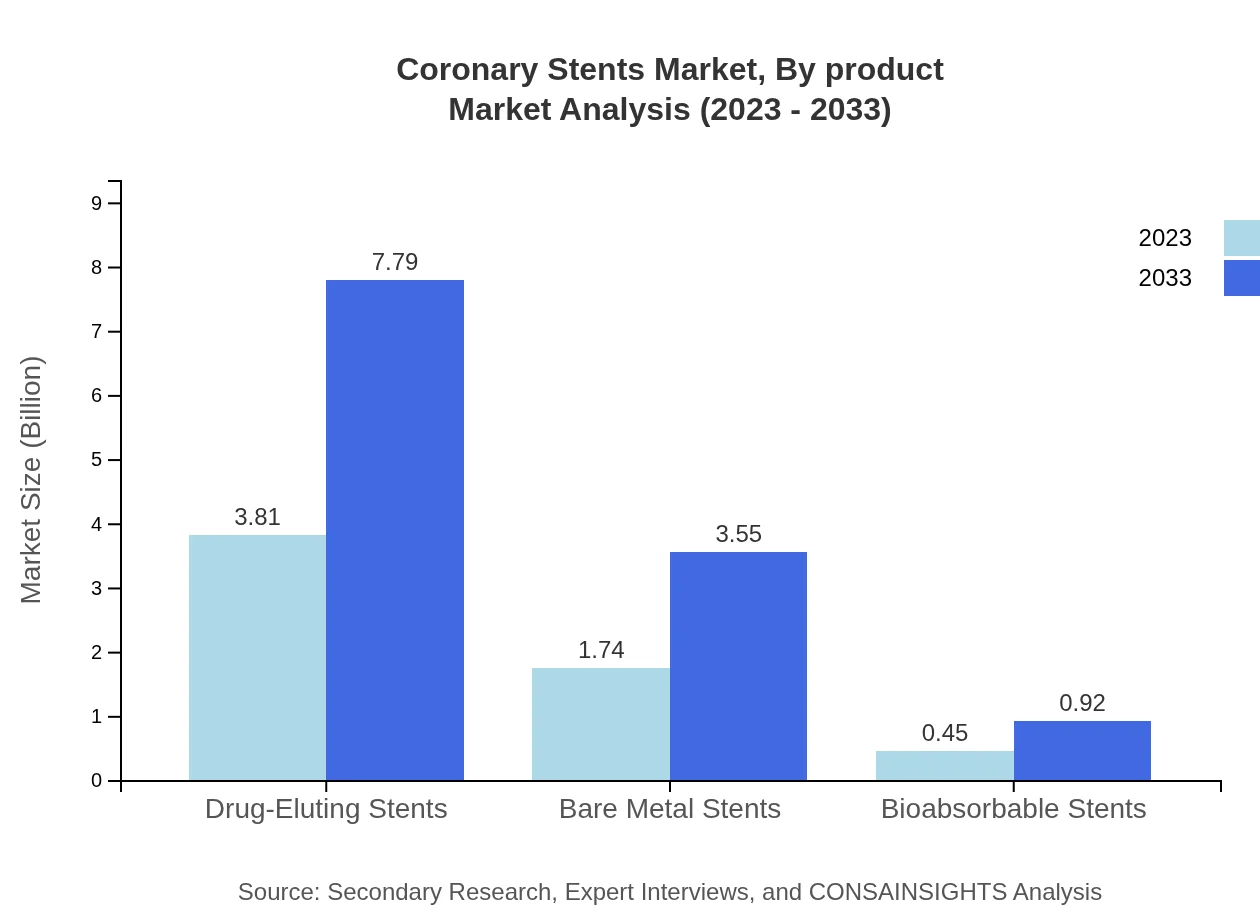
The market is segmented into three major types of stents: Drug-Eluting Stents (DES), Bare Metal Stents (BMS), and Bioabsorbable Stents (BAS). In 2023, Drug-Eluting Stents represented a significant market size of $3.81 billion, maintaining a share of 63.54%. This segment, benefiting from innovative drug delivery mechanisms, is projected to reach $7.79 billion by 2033. Bare Metal Stents, however, are estimated to grow from $1.74 billion to $3.55 billion during the same period. Bioabsorbable Stents, though currently smaller at $0.45 billion, may increase to $0.92 billion as technology advances.
Coronary Stents Market Analysis By Application
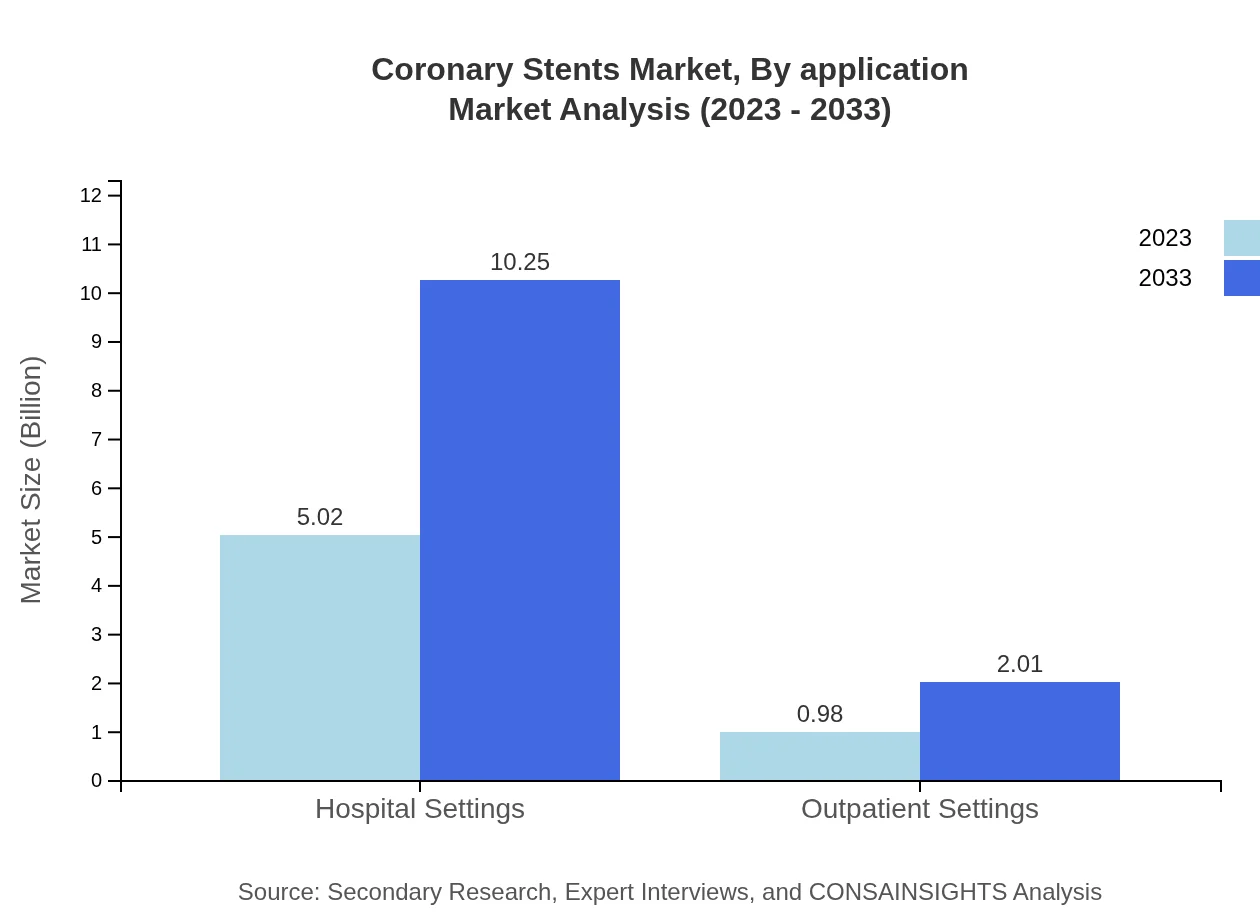
In terms of applications, the Coronary Stents market is diversified into several treatment pathways, particularly focusing on coronary artery disease management. Hospitals dominate this segment with a market share of 83.61%, valued at $5.02 billion in 2023 and expected to reach $10.25 billion by 2033. Cardiology clinics and outpatient settings, while smaller segments accounting for 16.39% of the share, also see considerable growth from $0.98 billion to $2.01 billion in the same timeframe.
Coronary Stents Market Analysis By End User
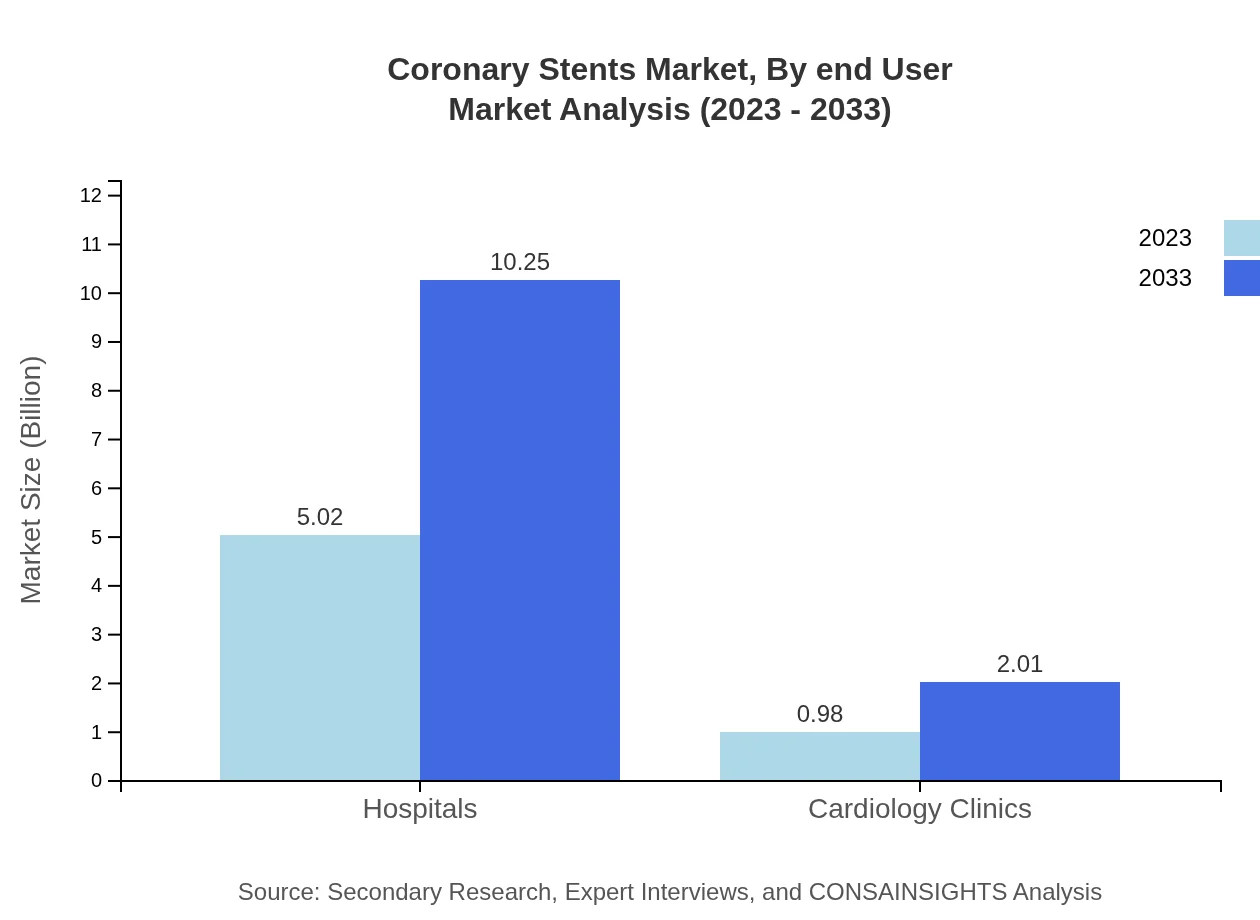
The Coronary Stents market segments by end-user include hospitals, outpatient surgical centers, and cardiology clinics. Hospitals account for a significant portion of the market, projected to increase substantially due to the rising number of heart surgeries. Outpatient settings, representing a growing trend in cardiovascular care, are expected to expand as the paradigm shifts toward less invasive procedures.
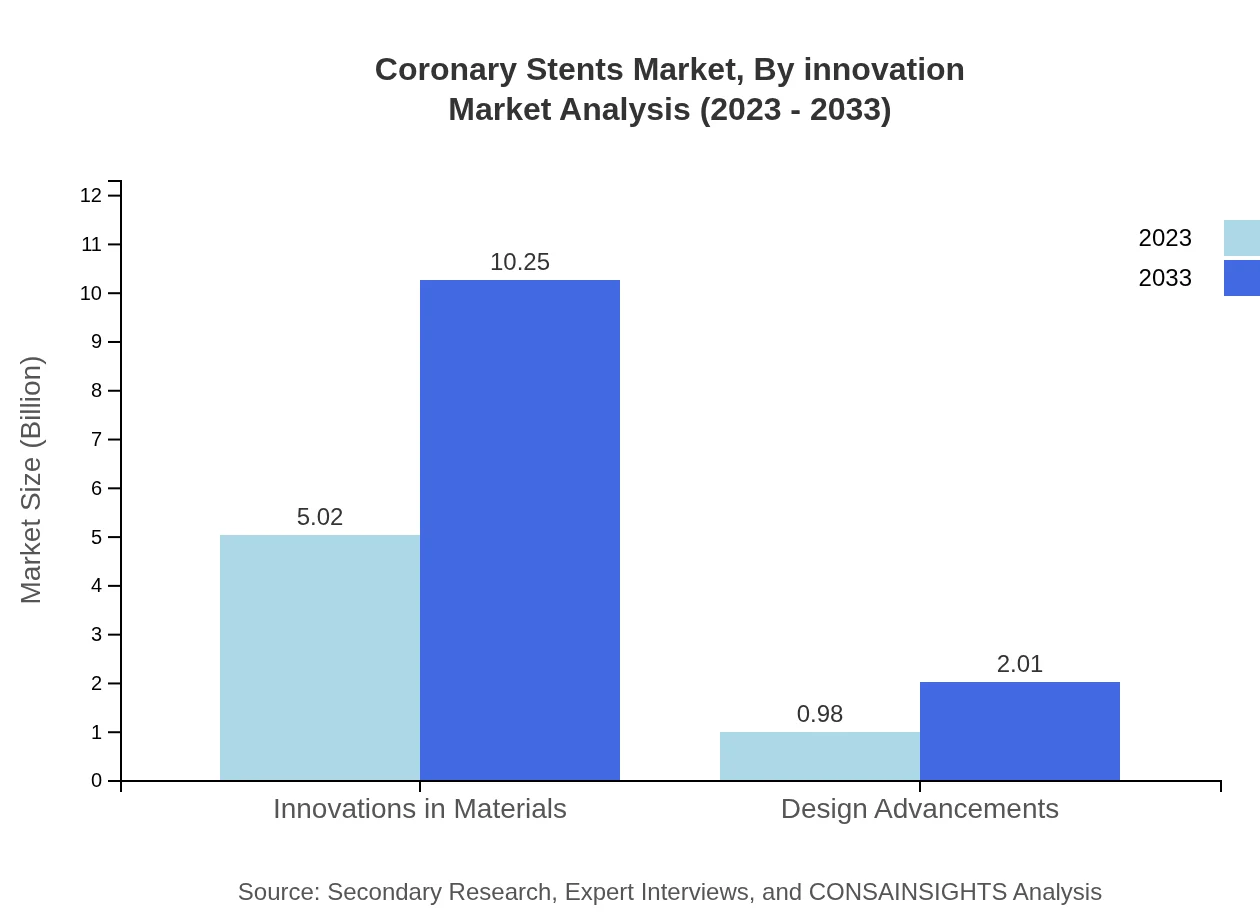
Coronary Stents Market Analysis By Innovation
Innovation is at the forefront of the Coronary Stents market, with advancements in stent materials, designs, and delivery systems. Innovations in materials, which account for a market size of $5.02 billion in hospital settings, leverage new biocompatible polymers, significantly reducing the incidence of thrombosis. Design advancements enhance the efficacy of stents in various clinical conditions, promoting patient recovery and reducing complications.
Coronary Stents Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Coronary Stents Industry
Medtronic :
Medtronic is a leader in medical devices and therapies across various areas, including cardiovascular health, developing innovative coronary stents that enhance patient outcomes.Abbott Laboratories:
Abbott is known for its advanced drug-eluting stents, contributing significantly to the market with cutting-edge technologies in coronary procedures.Boston Scientific:
Boston Scientific specializes in complex cardiovascular interventions and is recognized for its innovative coronary stent products that address challenging patient needs.B.Braun:
B.Braun focuses on vascular care and contributes to the market with its comprehensive range of stent technologies designed to improve cardiac health.Terumo Corporation:
Terumo is known for its commitment to patient safety and innovation in the coronary stent segment, providing high-quality solutions for interventional cardiology.We're grateful to work with incredible clients.









FAQs
What is the market size of coronary Stents?
The global coronary stents market is valued at approximately $6 billion in 2023, with an expected growth at a CAGR of 7.2%, projecting significant expansion through 2033.
What are the key market players or companies in this coronary Stents industry?
Key market players include major medical device companies that specialize in cardiovascular health, focusing on innovative stent designs and technologies to improve patient outcomes.
What are the primary factors driving the growth in the coronary Stents industry?
The growth is driven by increasing cardiovascular diseases prevalence, technological advancements in stent design, and rising healthcare expenditure globally, facilitating enhanced treatment options.
Which region is the fastest Growing in the coronary Stents?
The North American region is the fastest-growing, projected to expand from $1.97 billion in 2023 to $4.02 billion by 2033, driven by advanced healthcare systems and regulatory support.
Does ConsaInsights provide customized market report data for the coronary Stents industry?
Yes, ConsaInsights offers tailored market report data for the coronary-stents industry, catering to specific client needs and strategic decision-making.
What deliverables can I expect from this coronary Stents market research project?
Deliverables include comprehensive market analysis reports, insights on trends and forecasts, competitive landscape assessments, and specific data tailored to client requirements.
What are the market trends of coronary Stents?
Current trends include the growing adoption of drug-eluting stents and bioabsorbable stents, advancements in minimally invasive technologies, and increasing focus on innovation in materials used in stent manufacturing.