Counterfeit Drug Detection Device Market Report
Published Date: 31 January 2026 | Report Code: counterfeit-drug-detection-device
Counterfeit Drug Detection Device Market Size, Share, Industry Trends and Forecast to 2033
This report offers an in-depth analysis of the Counterfeit Drug Detection Device market from 2023 to 2033, encompassing insights on market size, trends, segmentations, and regional performances, alongside forecasts to guide stakeholders in strategic planning.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
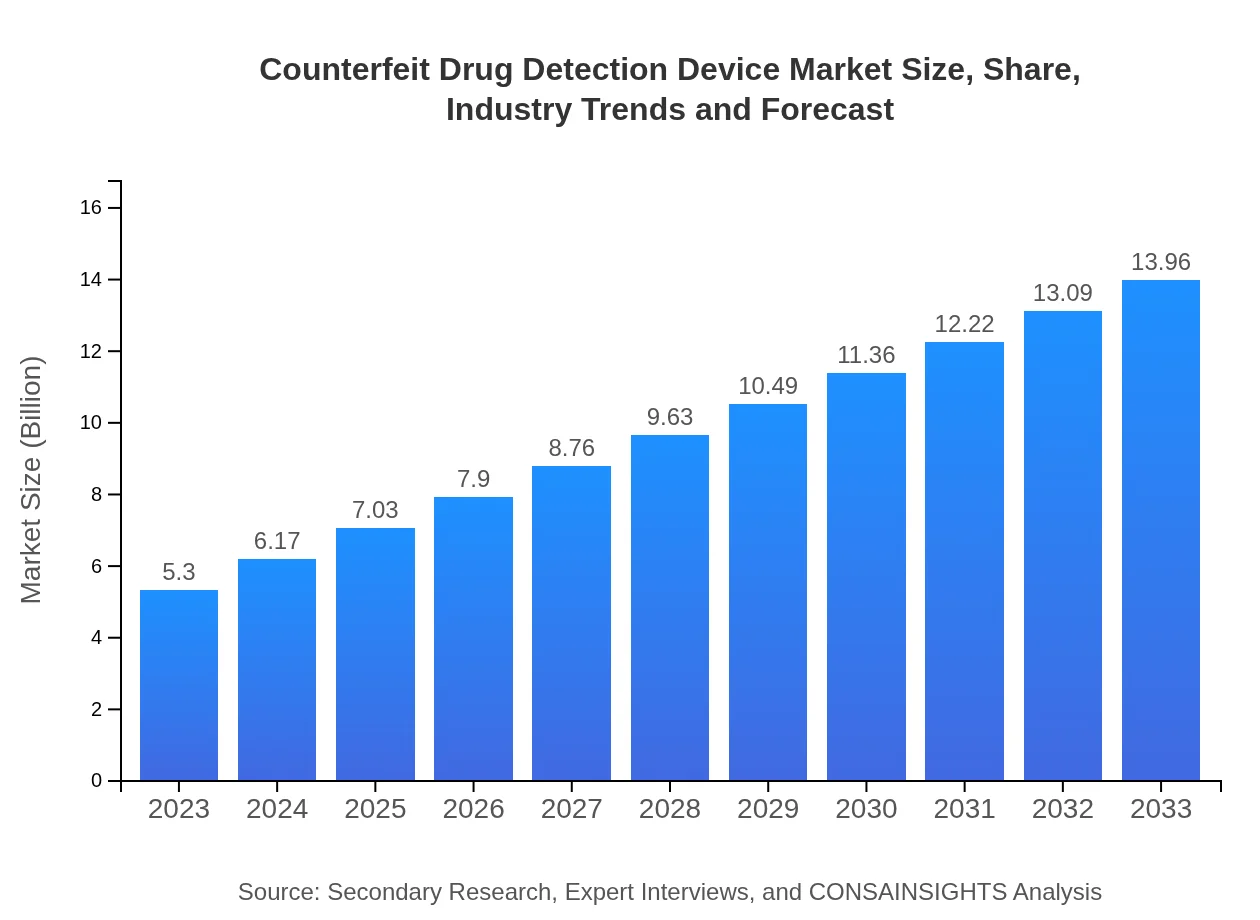
| 2023 Market Size | $5.30 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $13.96 Billion |
| Top Companies | Roche, Thermo Fisher, Agilent Technologies, Merck Group |
| Last Modified Date | 31 January 2026 |
Counterfeit Drug Detection Device Market Overview
Customize Counterfeit Drug Detection Device Market Report market research report
- ✔ Get in-depth analysis of Counterfeit Drug Detection Device market size, growth, and forecasts.
- ✔ Understand Counterfeit Drug Detection Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Counterfeit Drug Detection Device
What is the Market Size & CAGR of Counterfeit Drug Detection Device market in 2033?
Counterfeit Drug Detection Device Industry Analysis
Counterfeit Drug Detection Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Counterfeit Drug Detection Device Market Analysis Report by Region
Europe Counterfeit Drug Detection Device Market Report:
Europe's Counterfeit Drug Detection Device market is anticipated to expand significantly, from $1.76 billion in 2023 to $4.64 billion by 2033. The EU has implemented strict guidelines which promote the use of these detection devices, enhancing market opportunities in pharmaceutical sectors.Asia Pacific Counterfeit Drug Detection Device Market Report:
The Asia Pacific region is witnessing significant growth due to rising awareness around counterfeit drugs and increased healthcare expenditure. The market size is projected to reach $2.65 billion by 2033, up from $1.01 billion in 2023. Countries like India and China are leading adoption rates, supported by growing pharmaceutical industries and stringent regulations.North America Counterfeit Drug Detection Device Market Report:
North America holds a prominent share, projected to grow from $1.75 billion in 2023 to $4.60 billion in 2033. This growth is driven by stringent regulatory frameworks alongside high consumer awareness around counterfeit drugs, making robust detection technology imperative.South America Counterfeit Drug Detection Device Market Report:
The South American market is expanding gradually, with the market size forecasted to increase from $0.49 billion in 2023 to $1.28 billion by 2033. Governments in the region are beginning to focus more on healthcare safety, although the investment levels remain lower compared to more developed regions.Middle East & Africa Counterfeit Drug Detection Device Market Report:
The market in the Middle East and Africa is set to grow from $0.30 billion in 2023 to $0.78 billion by 2033. Emerging economies in the region are beginning to recognize the importance of drug safety, though challenges remain due to infrastructural constraints.Tell us your focus area and get a customized research report.
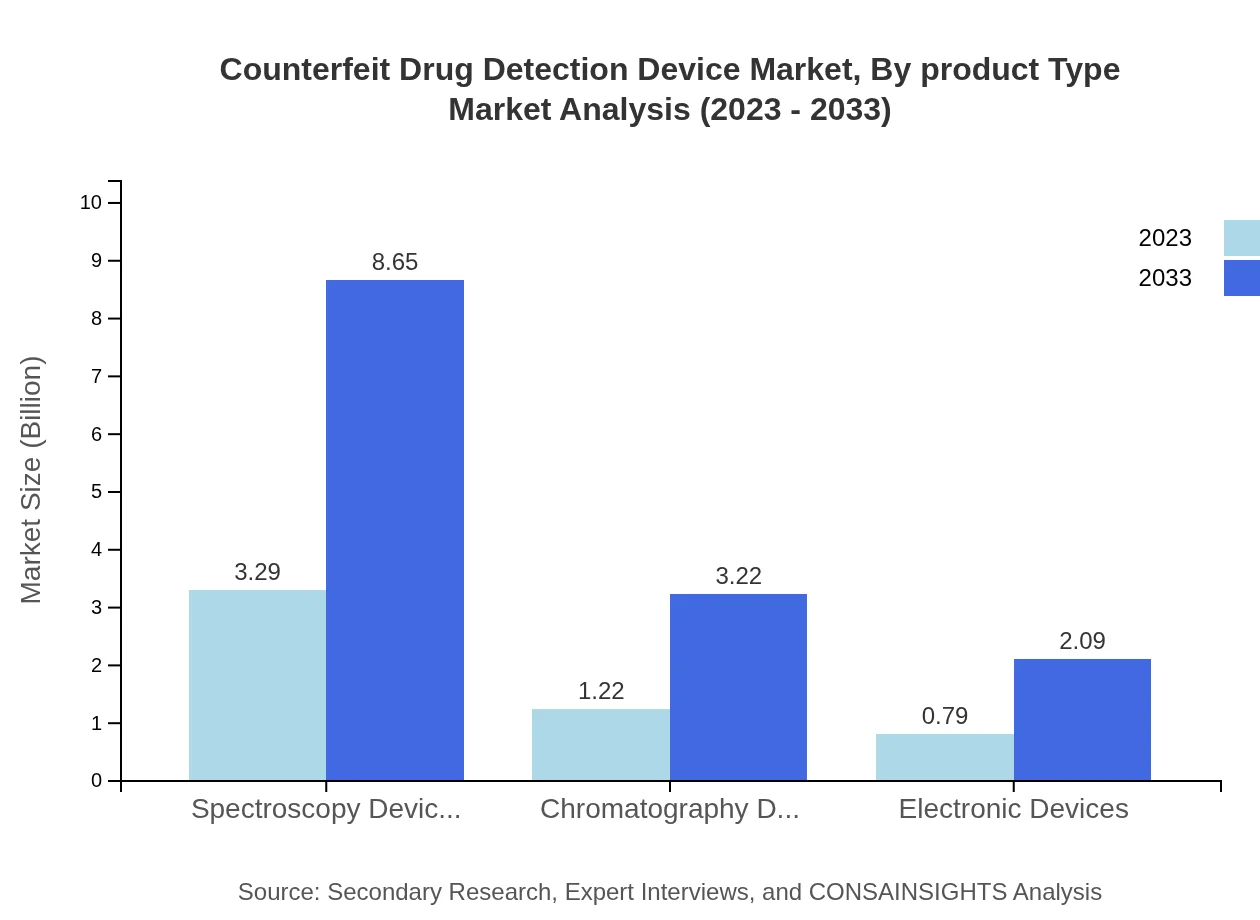
Counterfeit Drug Detection Device Market Analysis By Product Type
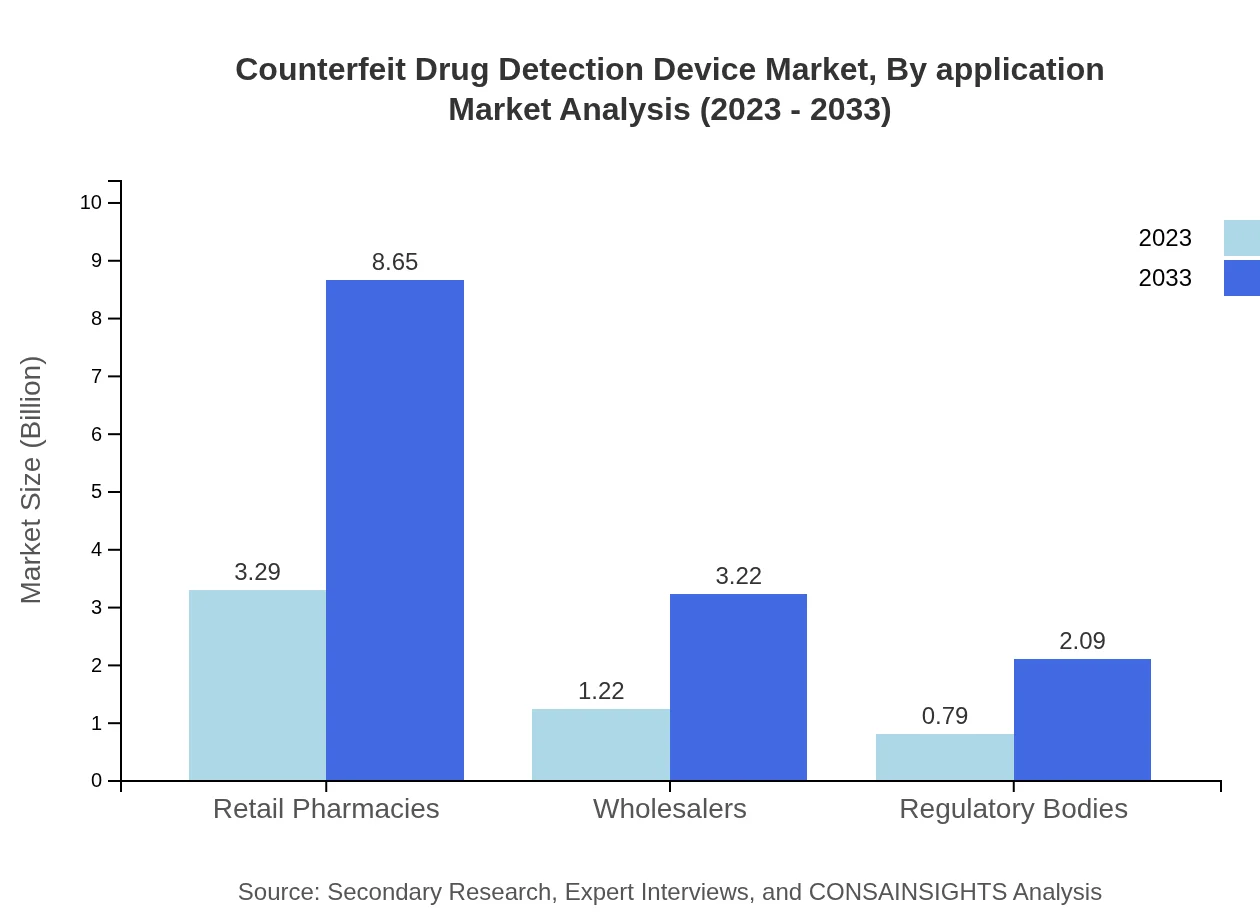
The product segmentation reveals that spectroscopy devices, listed at $3.29 billion in 2023, are the most significant contributor to the market, expected to reach $8.65 billion by 2033. Chromatography devices follow, increasing from $1.22 billion to $3.22 billion within the same timeframe. Continuous innovation in these technologies is driving their demand across pharmaceutical companies.
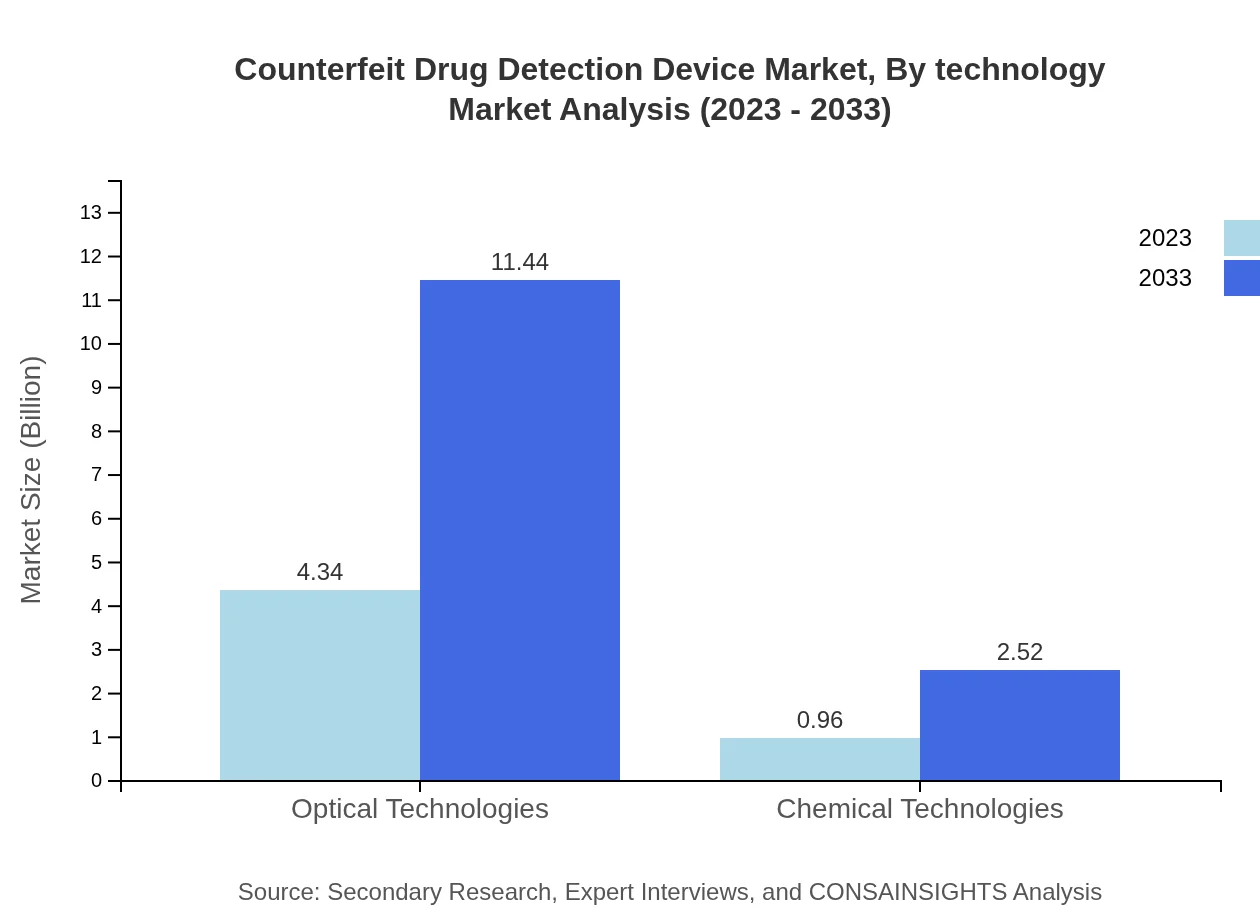
Counterfeit Drug Detection Device Market Analysis By Technology
Optical technologies dominate the market, with a significant market share of 81.97%. The segment size is projected to increase from $4.34 billion in 2023 to $11.44 billion by 2033. Chemical technologies have a smaller share but are gaining traction due to new developments, expected to grow from $0.96 billion to $2.52 billion.
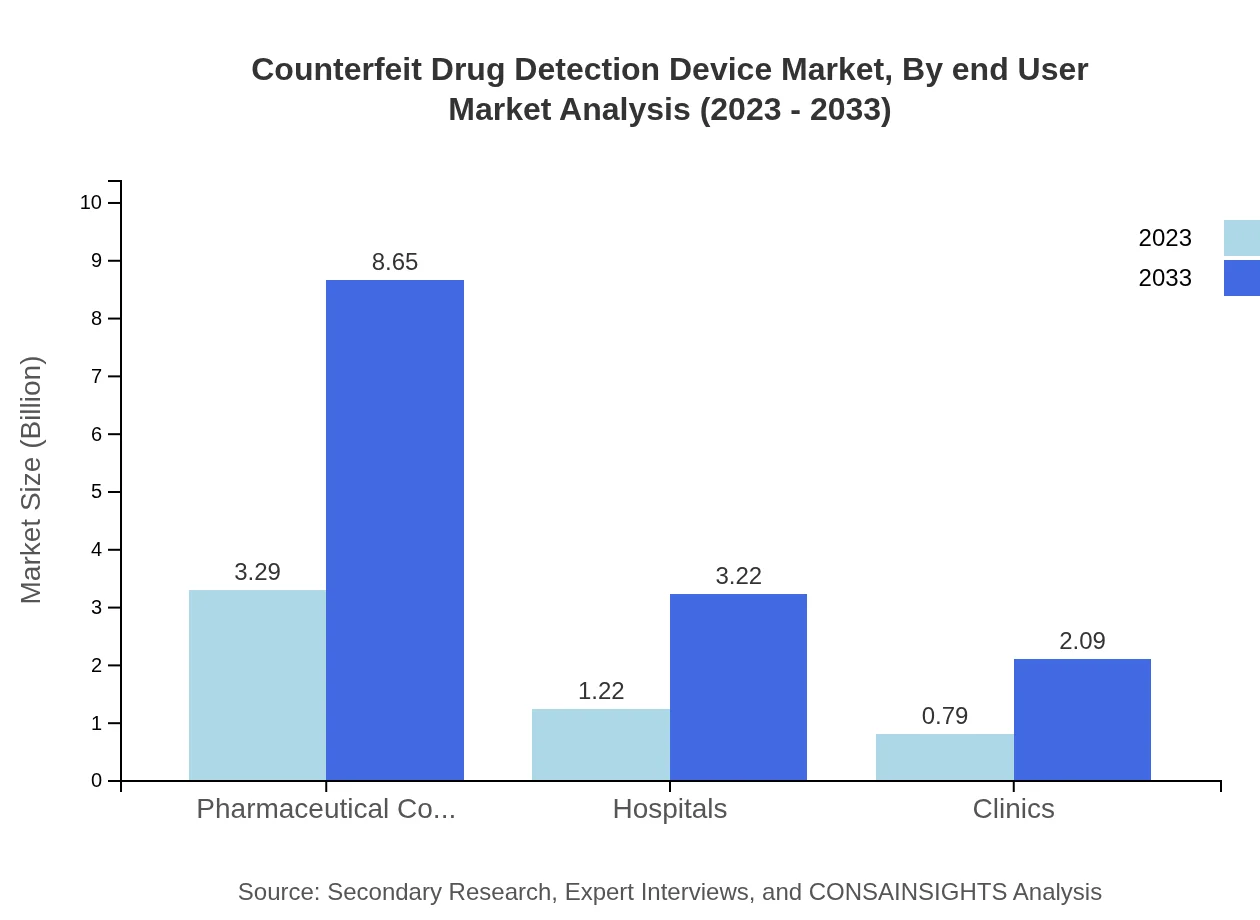
Counterfeit Drug Detection Device Market Analysis By End User
Pharmaceutical companies are leading in the adoption of detection devices, accumulating a market size of $3.29 billion in 2023 and expected to rise to $8.65 billion by 2033. Hospitals and clinics also play pivotal roles, emphasizing the necessity for robust drug verification systems to ensure patient safety.
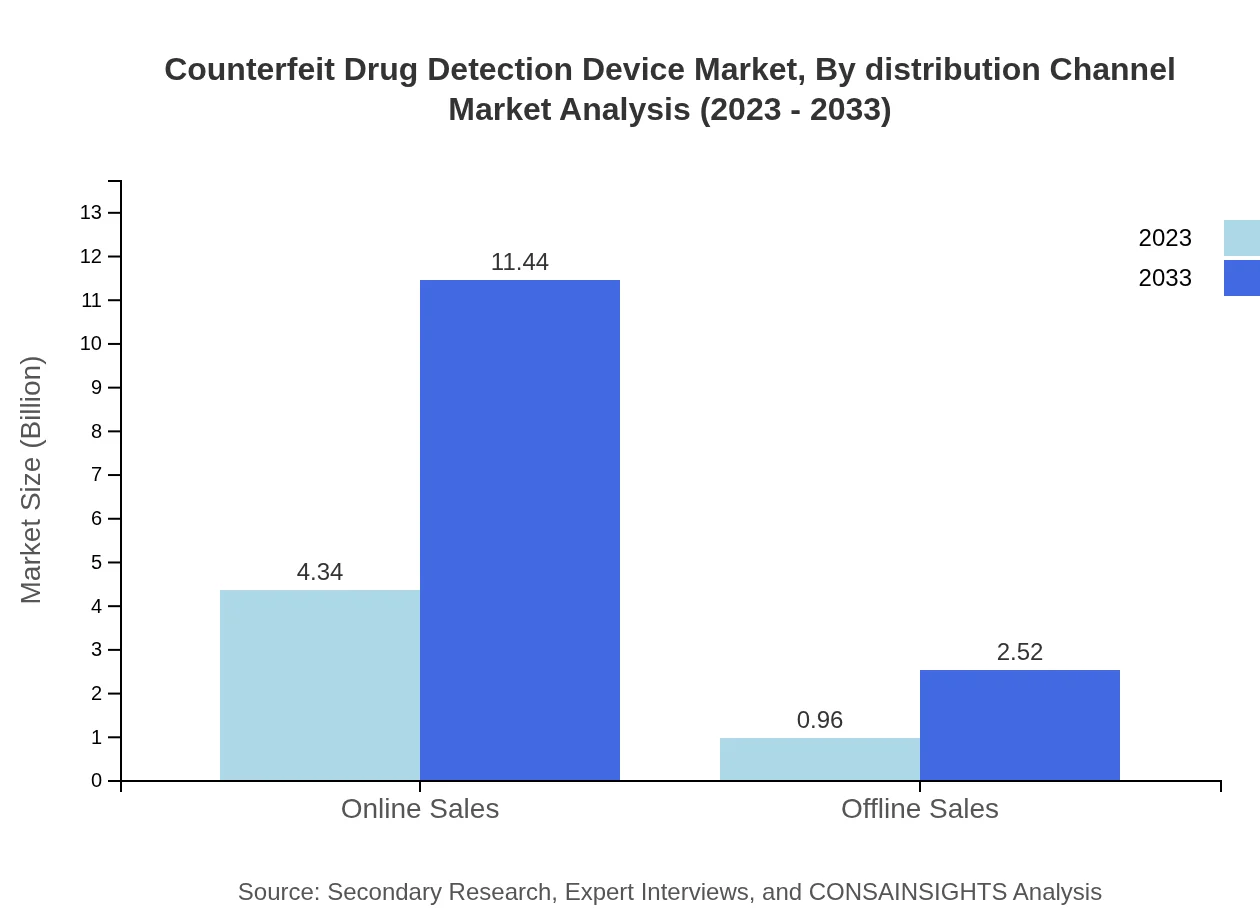
Counterfeit Drug Detection Device Market Analysis By Distribution Channel
Online sales channels dominate the distribution segment, holding an 81.97% share with a projected growth from $4.34 billion to $11.44 billion. Offline channels remain significant, yet show slower growth from $0.96 billion to $2.52 billion, indicating a shift towards e-commerce in drug detection solutions.
Counterfeit Drug Detection Device Market Analysis By Application
Applications in regulatory bodies and hospitals are increasingly emphasizing the need for detection devices. With market sizes expected to increase significantly by 2033, application in regulatory verification continues to drive innovation across the sector, optimizing safety protocols.
Counterfeit Drug Detection Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Counterfeit Drug Detection Device Industry
Roche:
Roche is a leading player in the pharmaceutical industry, investing heavily in advanced detection technologies to ensure drug authenticity and efficacy.Thermo Fisher:
Thermo Fisher is renowned for its innovative laboratory solutions, providing advanced counterfeit detection systems that are widely adopted in hospitals and labs.Agilent Technologies:
Agilent Technologies specializes in analytical instruments and solutions, playing a pivotal role in developing highly efficient counterfeiting detection devices.Merck Group:
Merck Group is committed to safe healthcare practices, focusing on drug authenticity through its range of counterfeit detection solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of counterfeit Drug Detection Device?
The counterfeit drug detection device market is projected to grow from $5.3 billion in 2023 to significant heights by 2033, showcasing a robust compound annual growth rate (CAGR) of 9.8%. This consistent growth indicates a rising demand for reliable pharmaceutical safety measures.
What are the key market players or companies in this counterfeit Drug Detection Device industry?
Key players in the counterfeit drug detection device industry include prominent pharmaceutical companies, technological innovators specializing in detection devices, and regulatory bodies. These entities collaborate to advance anti-counterfeiting technologies, ensuring drug integrity and patient safety.
What are the primary factors driving the growth in the counterfeit Drug Detection Device industry?
Growth in the counterfeit drug detection device industry is driven primarily by increasing global incidence of counterfeit medicines, regulatory pressure for compliance, technological advancements in detection devices, and heightened awareness surrounding patient safety across healthcare systems.
Which region is the fastest Growing in the counterfeit Drug Detection Device?
The fastest-growing region in the counterfeit drug detection device market is Europe, with market size projections increasing from $1.76 billion in 2023 to $4.64 billion by 2033. This surge is attributed to stringent regulations and a proactive stance against counterfeiting practices.
Does ConsaInsights provide customized market report data for the counterfeit Drug Detection Device industry?
Yes, ConsaInsights offers customized market report data for the counterfeit drug detection device industry. Clients can receive tailored insights, focusing on specific segments, regions, and emerging trends that align with their strategic objectives.
What deliverables can I expect from this counterfeit Drug Detection Device market research project?
From the counterfeit drug detection device market research project, clients can expect comprehensive reports featuring market size analysis, growth trends, competitive landscape assessments, regional insights, and detailed segmentation data tailored to their specific needs.
What are the market trends of counterfeit Drug Detection Device?
Current market trends in counterfeit drug detection devices include a shift towards integrated technological solutions, increasing adoption of online sales channels, and a focus on advanced detection methods. These trends signify a proactive approach to combating counterfeit drugs globally.