Covid-19 Detection Kits Market Report
Published Date: 31 January 2026 | Report Code: covid-19-detection-kits
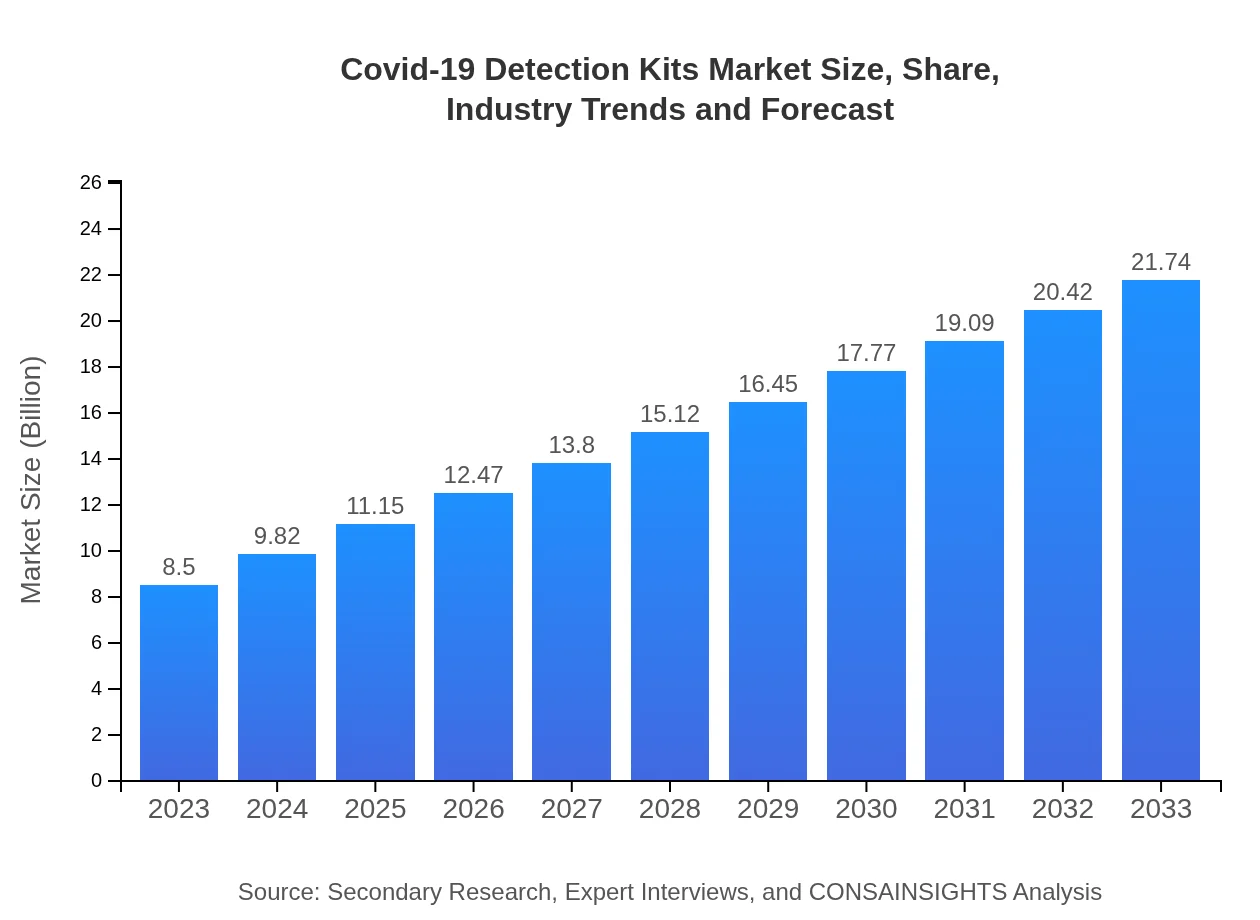
Covid-19 Detection Kits Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Covid-19 Detection Kits market from 2023 to 2033, offering insights into market size, growth trends, regional dynamics, and competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $8.50 Billion |
| CAGR (2023-2033) | 9.5% |
| 2033 Market Size | $21.74 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, Becton Dickinson |
| Last Modified Date | 31 January 2026 |
Covid-19 Detection Kits Market Overview
Customize Covid-19 Detection Kits Market Report market research report
- ✔ Get in-depth analysis of Covid-19 Detection Kits market size, growth, and forecasts.
- ✔ Understand Covid-19 Detection Kits's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Covid-19 Detection Kits
What is the Market Size & CAGR of Covid-19 Detection Kits market in 2023?
Covid-19 Detection Kits Industry Analysis
Covid-19 Detection Kits Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Covid-19 Detection Kits Market Analysis Report by Region
Europe Covid-19 Detection Kits Market Report:
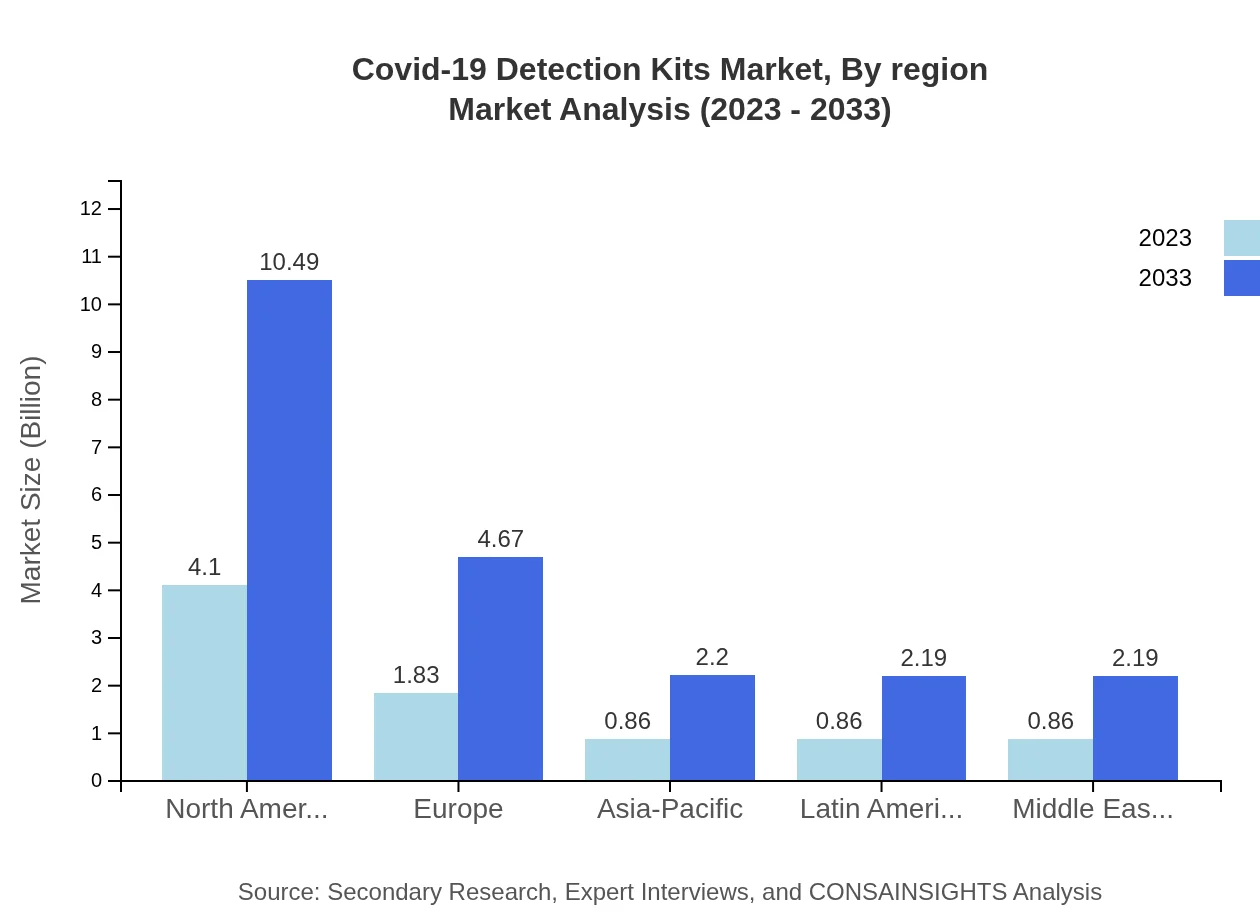
The European market, expected to grow from $2.22 billion in 2023 to $5.67 billion in 2033, benefits from stringent regulatory frameworks and active participation by governments in promoting widespread testing. The focus is on maintaining readiness for potential future outbreaks.Asia Pacific Covid-19 Detection Kits Market Report:
In the Asia Pacific region, the Covid-19 Detection Kits market is poised for considerable growth, expected to rise from $1.81 billion in 2023 to $4.63 billion by 2033. Countries like India and China are prioritizing testing with public health investments, thereby increasing market penetration for detection kits.North America Covid-19 Detection Kits Market Report:
North America holds a significant share of the market, with estimated values of $3.19 billion in 2023 and a projected growth to $8.17 billion in 2033. The demand is primarily driven by advanced healthcare systems, continuous testing strategies, and technological innovations.South America Covid-19 Detection Kits Market Report:
The South American market, starting from $0.59 billion in 2023, is projected to expand to $1.52 billion by 2033, bolstered by public health initiatives aimed at managing Covid-19 outbreaks and improving health infrastructure. However, challenges in distribution and varying regulatory frameworks remain concerns.Middle East & Africa Covid-19 Detection Kits Market Report:
The Middle East and Africa market, though smaller, is projected to grow from $0.69 billion in 2023 to $1.75 billion by 2033. Efforts to improve healthcare delivery and the establishment of testing facilities drive this growth, albeit at a slower pace compared to other regions.Tell us your focus area and get a customized research report.
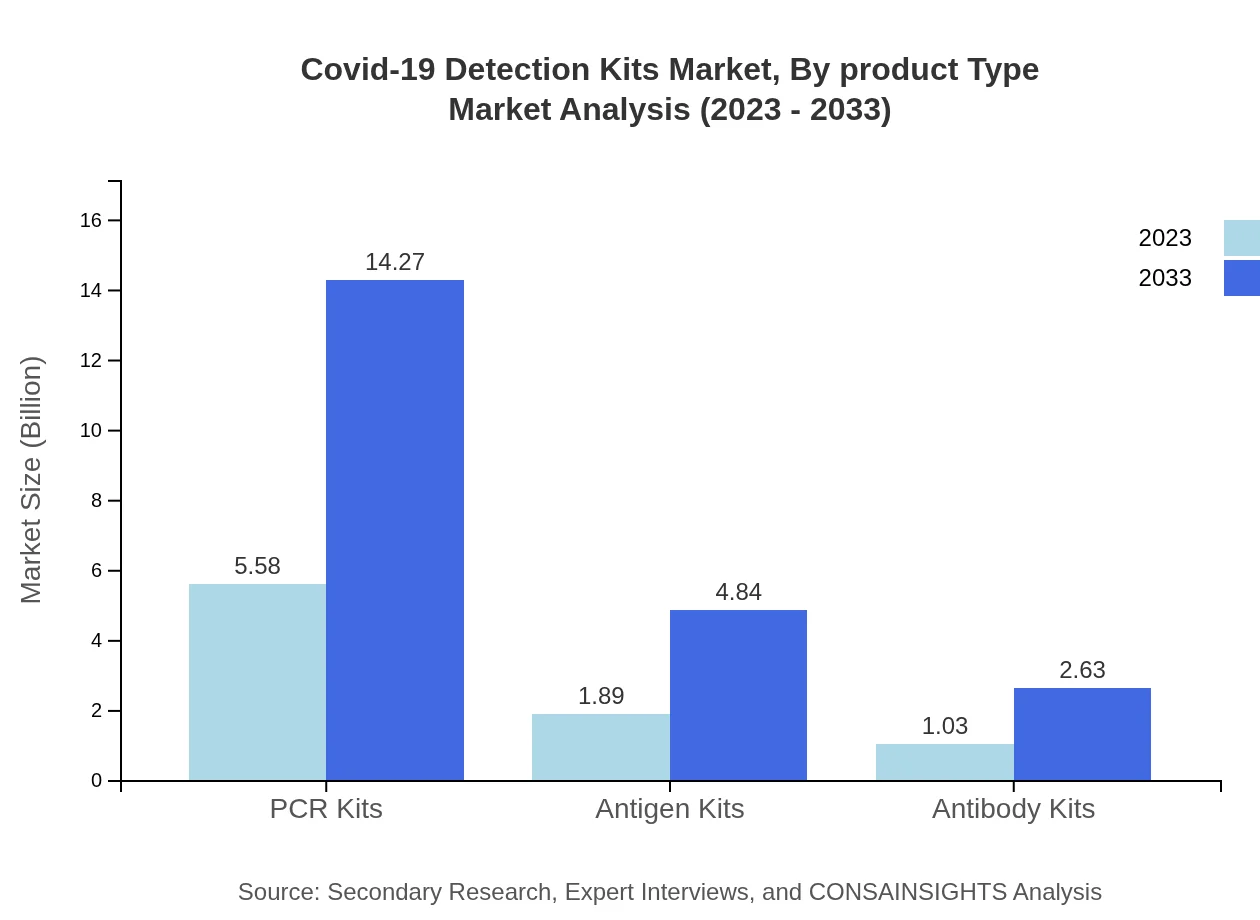
Covid-19 Detection Kits Market Analysis By Product Type
The market for Covid-19 detection kits is dominated by PCR kits significantly, estimated at $5.58 billion in 2023. Antigen kits are also critical, valued at $1.89 billion, while antibody kits are smaller in scale at $1.03 billion but vital for seroprevalence studies. The ongoing need for reliable testing solutions makes these product segments essential for market sustainability.
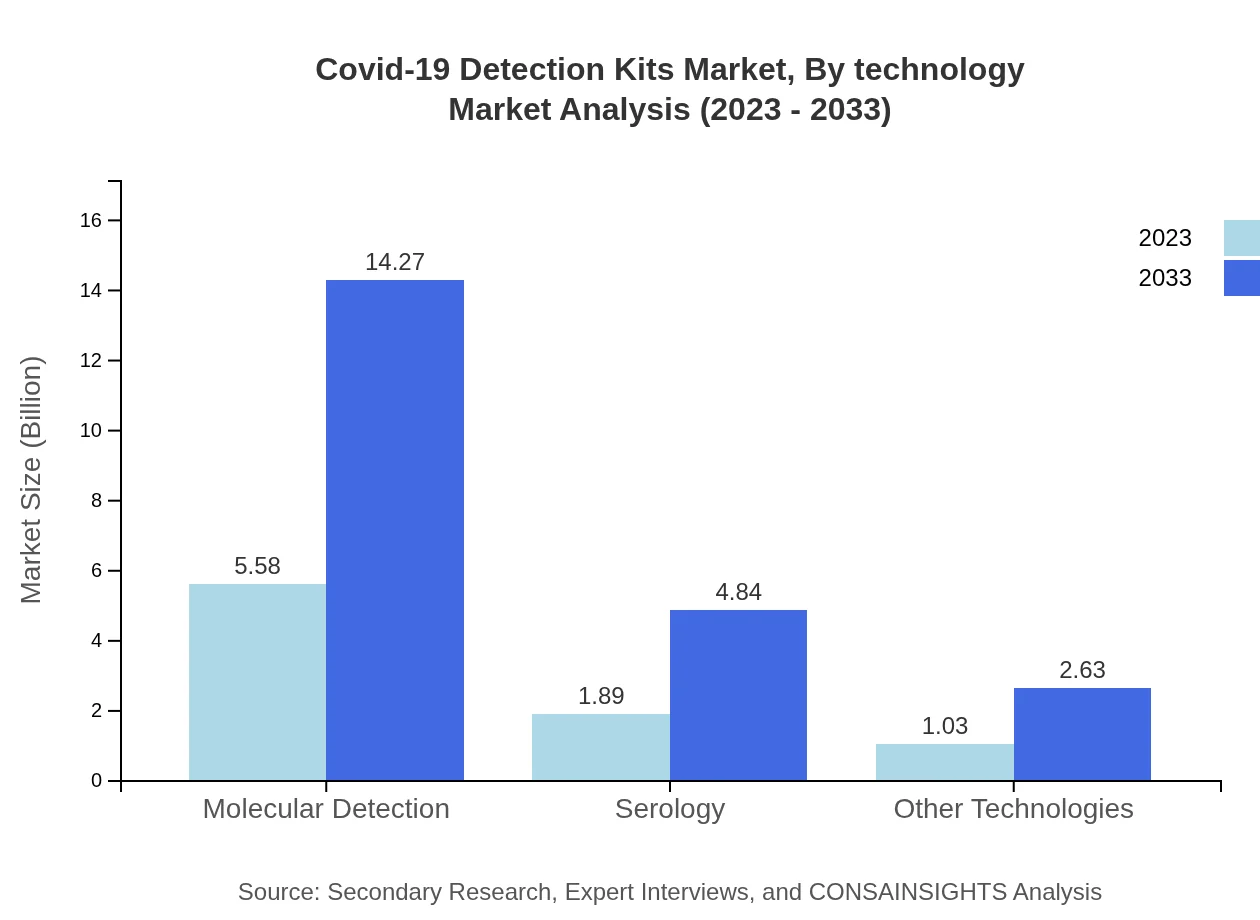
Covid-19 Detection Kits Market Analysis By Technology
Molecular detection technology leads the market with a $5.58 billion valuation in 2023, owing to its accuracy and reliability. Serology tests follow, representing a growing interest in understanding immune responses, valued at $1.89 billion. Other technologies account for $1.03 billion, reflecting diverse testing approaches and options available for users.
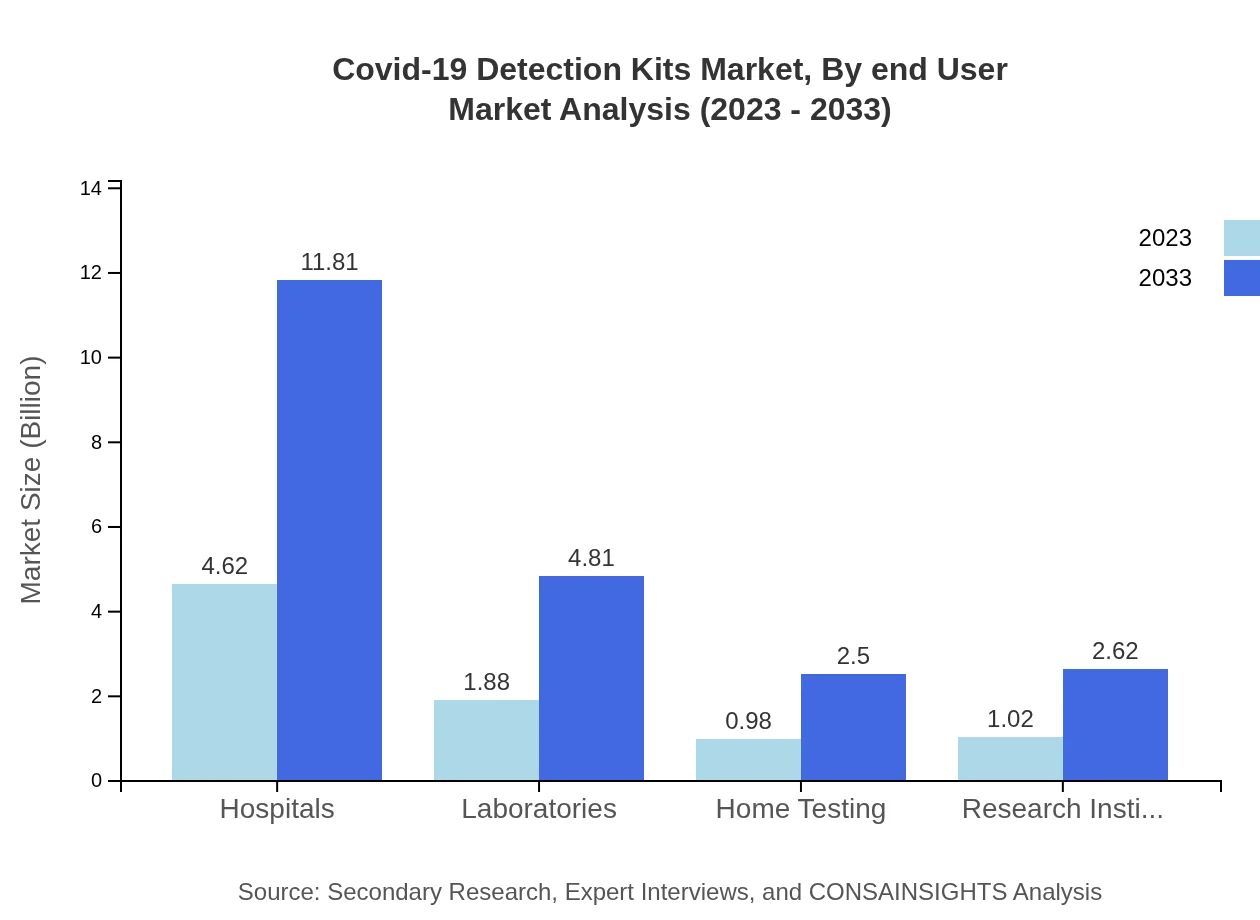
Covid-19 Detection Kits Market Analysis By End User
Hospitals constitute the largest end-user segment, valued at $4.62 billion in 2023, emphasizing the need for quick and reliable testing in clinical environments. Laboratories and research institutes are significant contributors as well, with total market shares of $1.88 billion and $1.02 billion respectively. Home testing is another growing segment with increasing consumer acceptance.
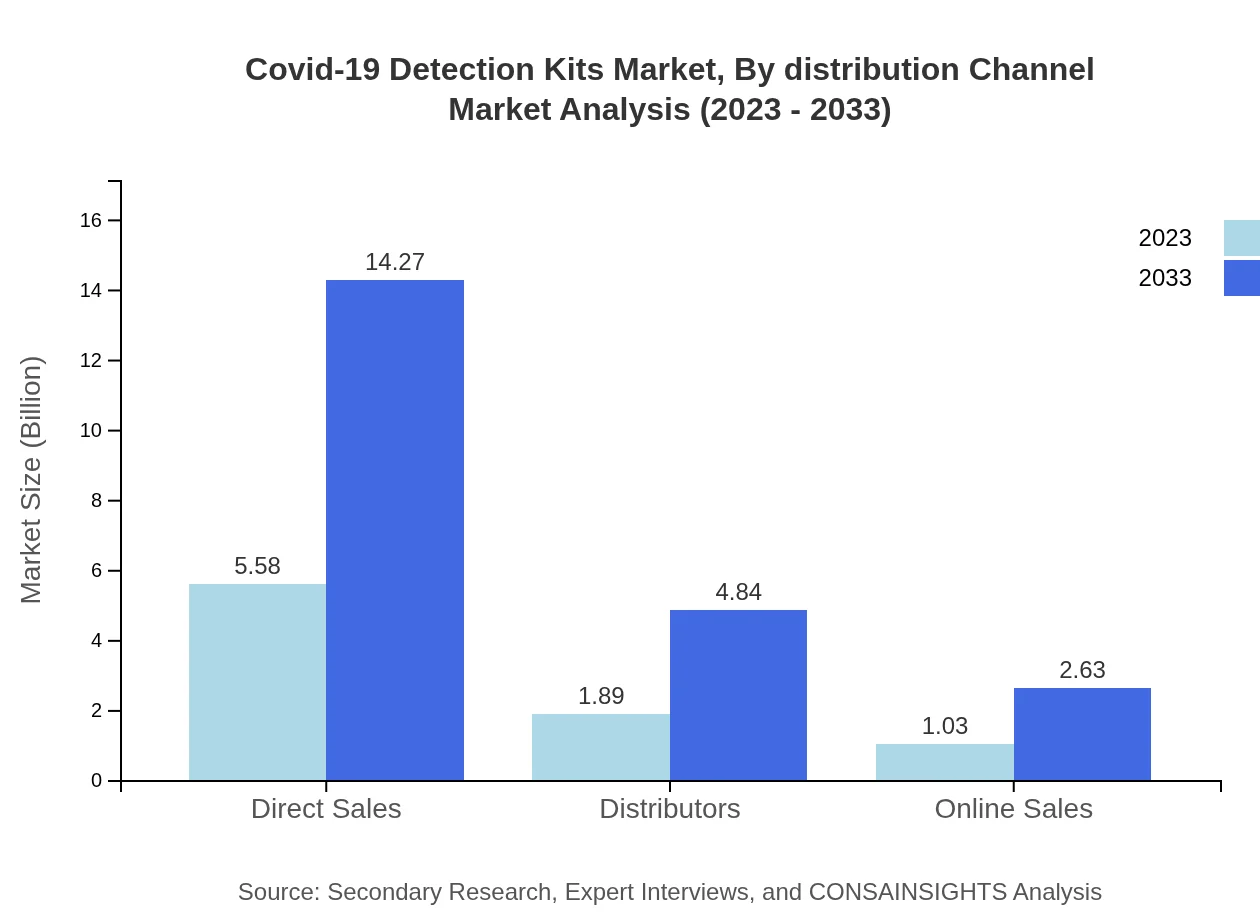
Covid-19 Detection Kits Market Analysis By Distribution Channel
Direct sales dominate the distribution channel landscape, accounting for $5.58 billion in 2023. Distributors play a critical role as well, contributing to $1.89 billion. Online sales, while smaller at $1.03 billion, are emerging as a significant channel, supported by increasing digital health solutions and consumer engagement.
Covid-19 Detection Kits Market Analysis By Region
The regional analysis highlights differing growth trends and market values, crucial for strategic planning in the Covid-19 Detection Kits industry. The North American region showcases the highest market growth, while emerging markets in Asia Pacific and South America exhibit rapid expansion potential, contributing to the global landscape.
Covid-19 Detection Kits Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Covid-19 Detection Kits Industry
Abbott Laboratories:
A leading company known for their rapid and accurate Covid-19 testing solutions. Abbott has developed various kits that have become essential in both clinical and at-home testing.Roche Diagnostics:
A global pioneer in diagnostics, Roche offers a wide range of Covid-19 detection kits that leverage innovative technology, enhancing testing efficiency and accuracy.Thermo Fisher Scientific:
A key player in the molecular diagnostics sector, Thermo Fisher has developed multiple Covid-19 detection solutions emphasizing high throughput and rapid results.Becton Dickinson:
Known for their advancements in medical technology, BD provides various Covid-19 detection kits, contributing to both global testing efforts and supply chains.We're grateful to work with incredible clients.









FAQs
What is the market size of covid-19 detection kits?
The global market size for Covid-19 detection kits is projected to reach approximately $8.5 billion by 2033, with a compound annual growth rate (CAGR) of 9.5% from its current valuation in 2023.
What are the key market players or companies in the covid-19 detection kits industry?
The Covid-19 detection kits market features major players such as Abbott Laboratories, Roche, Thermo Fisher Scientific, Siemens Healthineers, and BGI Genomics, leading innovation and market share through diverse product offerings.
What are the primary factors driving the growth in the covid-19 detection kits industry?
Factors contributing to the growth of the Covid-19 detection kits industry include increased testing demands, rising awareness of early diagnosis, expansion of healthcare infrastructure, and advancements in detection technologies.
Which region is the fastest Growing in the covid-19 detection kits?
Among the regions, North America emerged as the fastest-growing market for Covid-19 detection kits, expected to grow from $3.19 billion in 2023 to $8.17 billion by 2033, driven by high healthcare expenditure.
Does Consainsights provide customized market report data for the covid-19 detection kits industry?
Yes, Consainsights offers customized market report data tailored to specific requirements in the Covid-19 detection kits industry, ensuring clients receive relevant insights aligning with their strategic goals.
What deliverables can I expect from this covid-19 detection kits market research project?
Expect deliverables including comprehensive market analysis, growth forecasts, competitive landscape overviews, key market drivers, and segment breakdowns, facilitating informed strategic decisions within the Covid-19 detection kits market.
What are the market trends of covid-19 detection kits?
Current market trends in covid-19 detection kits include rising demand for rapid testing, shift towards home testing solutions, innovation in molecular detection technologies, and the integration of AI-driven diagnostic tools.