Covid19 Impact On Critical Care Device Market Report
Published Date: 31 January 2026 | Report Code: covid19-impact-on-critical-care-device
Covid19 Impact On Critical Care Device Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive report analyzes the Covid19 impact on the critical care device market, providing market insights, industry trends, and forecasts from 2023 to 2033. It offers detailed segmentation, regional analyses, and insights into technology advancements influencing market dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
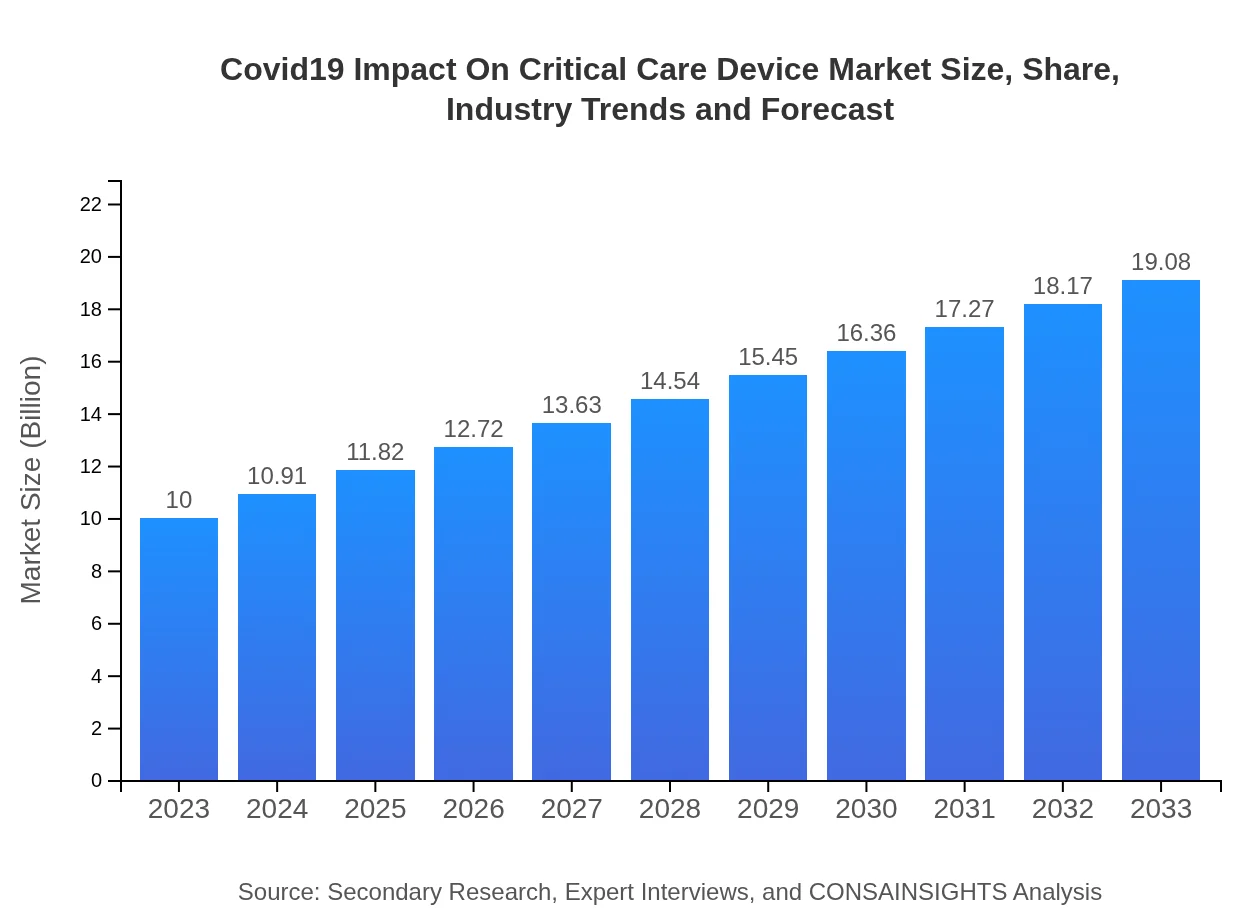
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $19.08 Billion |
| Top Companies | Philips Healthcare, Medtronic , GE Healthcare, Siemens Healthineers, Becton, Dickinson and Company |
| Last Modified Date | 31 January 2026 |
Covid19 Impact On Critical Care Device Market Overview
Customize Covid19 Impact On Critical Care Device Market Report market research report
- ✔ Get in-depth analysis of Covid19 Impact On Critical Care Device market size, growth, and forecasts.
- ✔ Understand Covid19 Impact On Critical Care Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Covid19 Impact On Critical Care Device
What is the Market Size & CAGR of Covid19 Impact On Critical Care Device market in 2023?
Covid19 Impact On Critical Care Device Industry Analysis
Covid19 Impact On Critical Care Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Covid19 Impact On Critical Care Device Market Analysis Report by Region
Europe Covid19 Impact On Critical Care Device Market Report:
In Europe, the market will rise from $2.48 billion in 2023 to $4.74 billion by 2033, backed by supportive governmental initiatives, a high prevalence of respiratory conditions, and an emphasis on enhancing hospital critical care capacities.Asia Pacific Covid19 Impact On Critical Care Device Market Report:
In the Asia Pacific region, the market is projected to grow from $2.19 billion in 2023 to $4.18 billion by 2033. Factors contributing to this growth include rising healthcare expenditure, increasing prevalence of chronic diseases, and enhancements in healthcare infrastructure.North America Covid19 Impact On Critical Care Device Market Report:
North America is anticipated to see significant growth, from $3.79 billion in 2023 to $7.24 billion by 2033. This is fueled by advanced healthcare infrastructure, high adoption of innovative technologies, and a robust regulatory framework supporting medical device advancements.South America Covid19 Impact On Critical Care Device Market Report:
The South American market is expected to expand from $0.28 billion in 2023 to $0.53 billion by 2033. The growth is driven by increasing investments in healthcare, although challenges such as economic fluctuations and infrastructural disparities persist.Middle East & Africa Covid19 Impact On Critical Care Device Market Report:
The Middle East and African market is forecasted to increase from $1.25 billion in 2023 to $2.39 billion by 2033. The growth is attributed to improving healthcare systems, increased disease awareness, and support from international healthcare organizations.Tell us your focus area and get a customized research report.
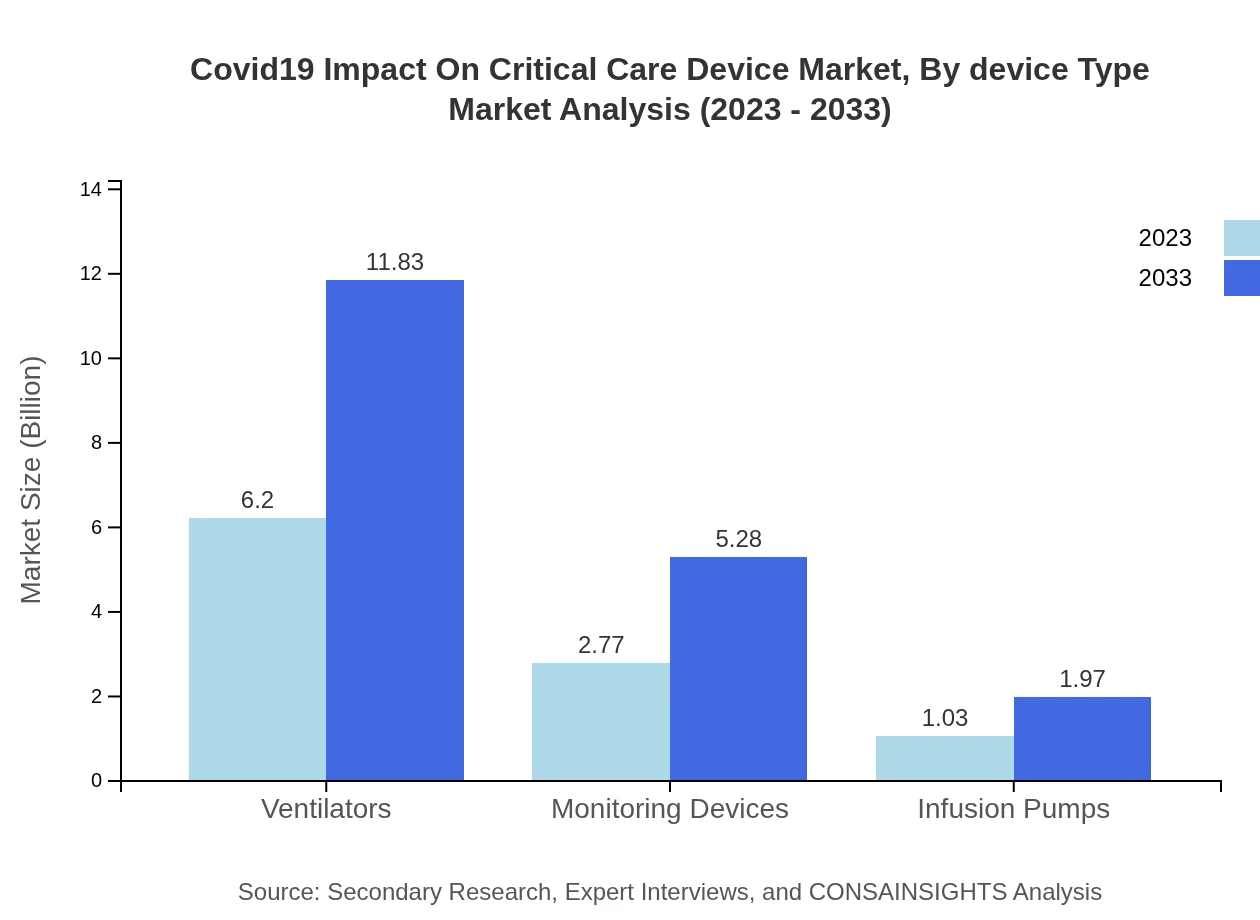
Covid19 Impact On Critical Care Device Market Analysis By Device Type
The market for critical care devices is predominantly segmented into ventilators, monitoring devices, infusion pumps, and others. Ventilators represent the largest share, with a market size increasing from $6.20 billion in 2023 to $11.83 billion by 2033. Monitoring devices, and infusion pumps follow, with substantial growth reflecting the increasing need for effective patient monitoring during critical care.
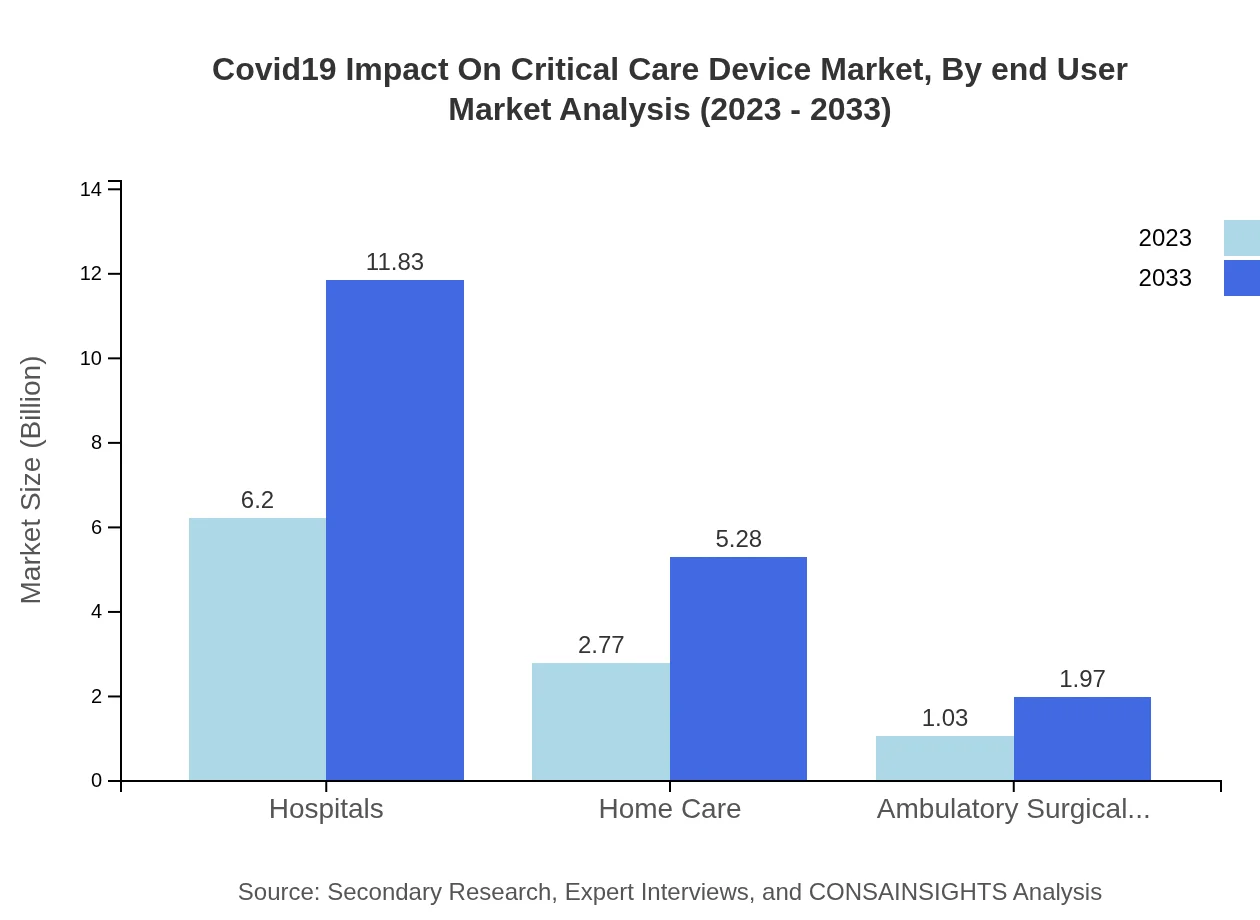
Covid19 Impact On Critical Care Device Market Analysis By End User
Hospitals are the leading end-user segment, accounting for a market share of 61.99% in 2023 and maintaining the same percentage in 2033. Home care settings are also expected to see growth, with market size increasing from $2.77 billion in 2023 to $5.28 billion by 2033, indicating a shift towards at-home care solutions.
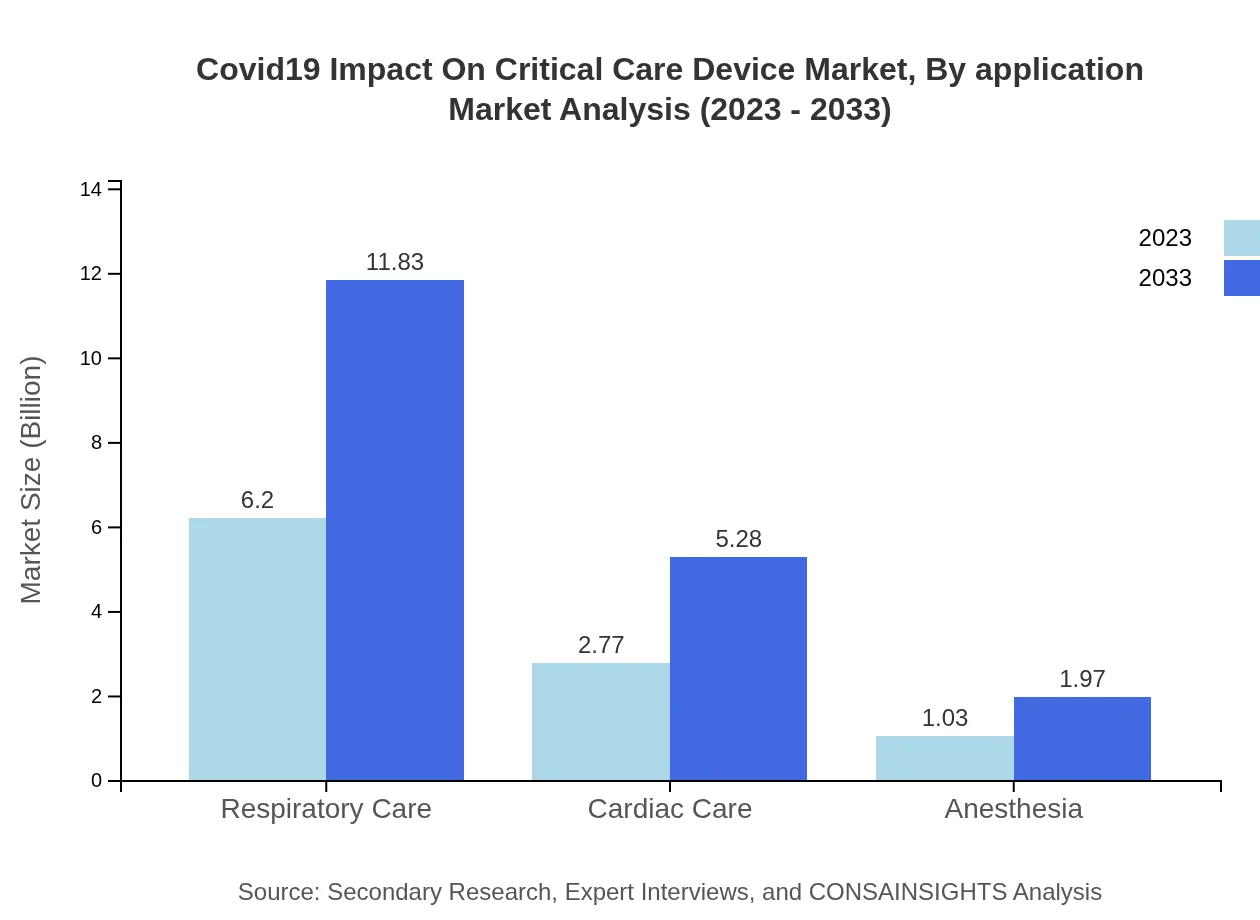
Covid19 Impact On Critical Care Device Market Analysis By Application
Critical care applications include respiratory care, cardiac care, anesthesia, and others. Respiratory care remains the most impactful segment, based on its critical role in treating Covid19 patients, constituting a market size of $6.20 billion in 2023 with an expected rise to $11.83 billion by 2033.
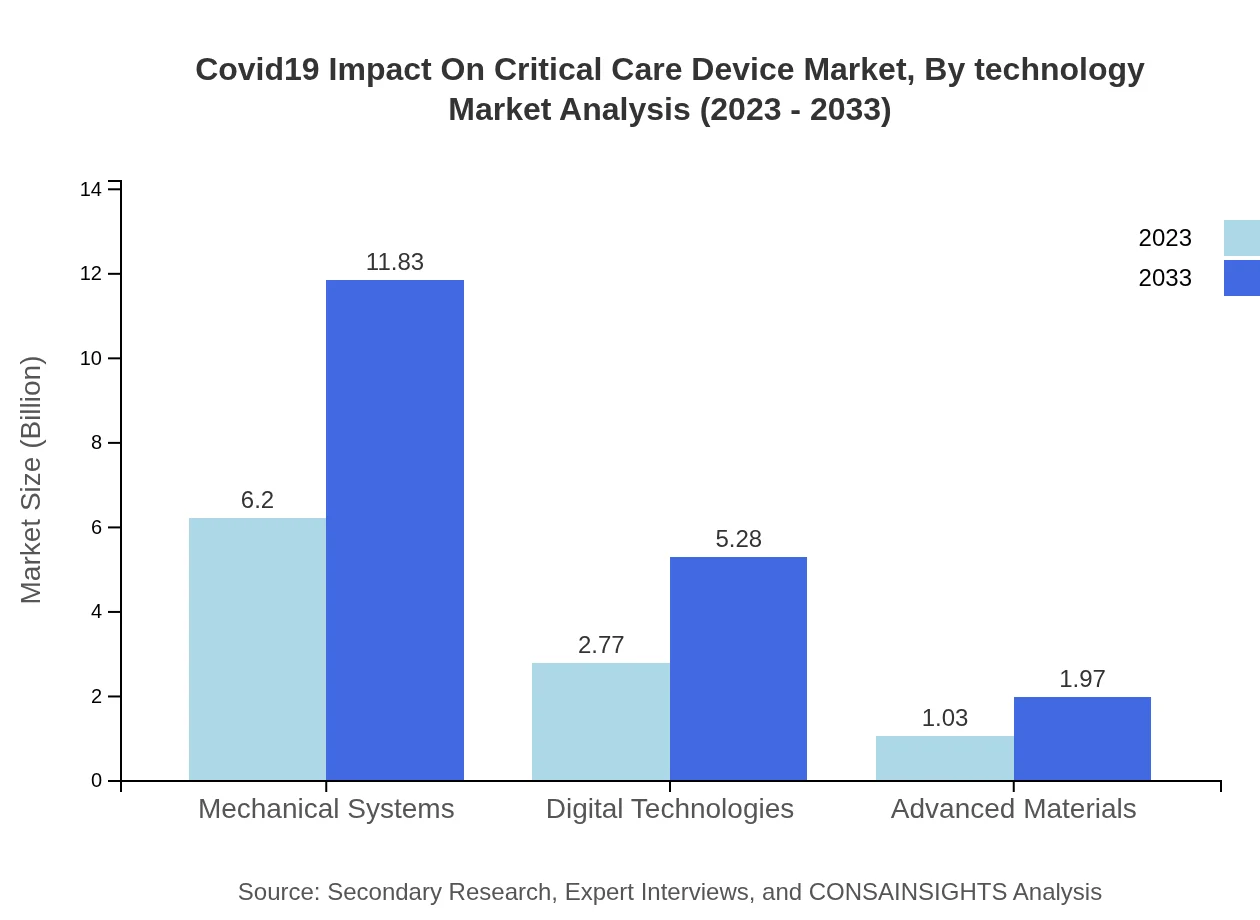
Covid19 Impact On Critical Care Device Market Analysis By Technology
Technological advancements are crucial in driving the market forward. Digital technologies are experiencing significant growth, projected to rise from $2.77 billion in 2023 to $5.28 billion by 2033. Innovations such as telemedicine integration and advanced monitoring systems have enhanced critical care delivery.
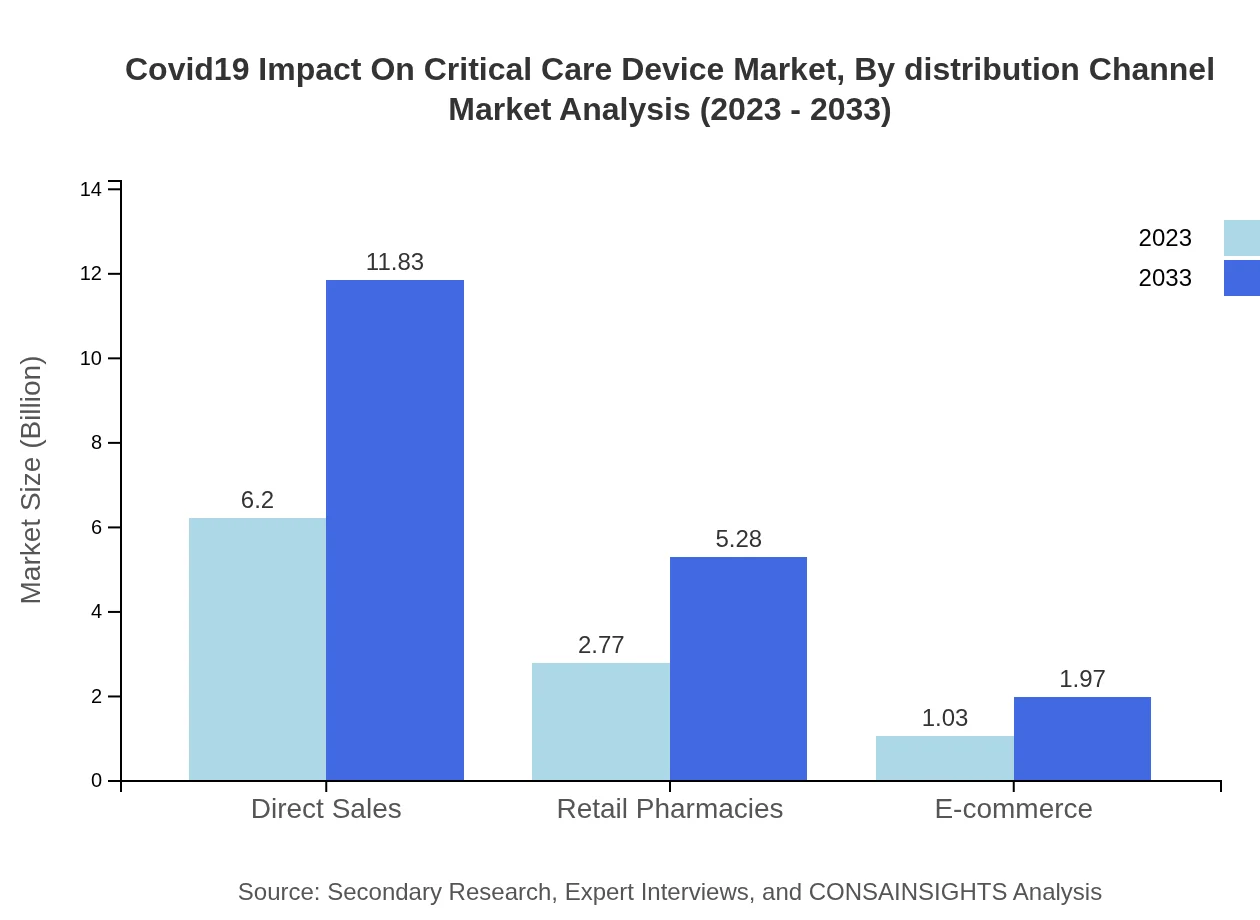
Covid19 Impact On Critical Care Device Market Analysis By Distribution Channel
Distribution channels for critical care devices include direct sales, retail pharmacies, and e-commerce platforms. Direct sales currently dominate the market, accounting for 61.99% in 2023 and expected to retain this share through 2033, while e-commerce is steadily gaining traction due to the growing trend of online shopping.
Covid19 Impact On Critical Care Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Covid19 Impact On Critical Care Device Industry
Philips Healthcare:
A leading global player in the healthcare market, Philips specializes in innovative medical devices, including highly advanced ventilators and monitoring systems used extensively during the Covid19 pandemic.Medtronic :
As a pioneer in medical technology, Medtronic provides a wide array of advanced critical care solutions, including respiratory care essentials that support patient recovery processes.GE Healthcare:
GE Healthcare's portfolio includes comprehensive critical care solutions, with innovative imaging and monitoring technologies that have proven crucial in managing Covid19 patients.Siemens Healthineers:
Siemens Healthineers is known for its robust diagnostic imaging and monitoring devices utilized in critical care, helping healthcare systems enhance patient outcomes.Becton, Dickinson and Company:
BD is focused on developing and delivering solutions for critical care, enhancing safety and quality in patient care through its advanced infusion technologies and monitoring systems.We're grateful to work with incredible clients.









FAQs
What is the market size of Covid19 Impact On Critical Care Device?
The Covid19 Impact On Critical Care Device market is projected to grow from a base market size of $10 billion in 2023, with a CAGR of 6.5% through 2033, indicating significant growth opportunities within this sector.
What are the key market players or companies in this Covid19 Impact On Critical Care Device industry?
Key players in the Covid19 Impact On Critical Care Device market include leading medical device manufacturers specializing in critical care technologies, though specific company names can be provided within detailed reports or upon request.
What are the primary factors driving the growth in the Covid19 Impact On Critical Care Device industry?
The growth of the Covid19 Impact On Critical Care Device market is driven by increased demand for critical care services, advancements in medical technology, and the need for efficient patient management solutions during health crises.
Which region is the fastest Growing in the Covid19 Impact On Critical Care Device?
North America is the fastest-growing region in the Covid19 Impact On Critical Care Device market, projected to expand from $3.79 billion in 2023 to $7.24 billion by 2033, leading in technological advancements and healthcare infrastructure.
Does ConsaInsights provide customized market report data for the Covid19 Impact On Critical Care Device industry?
Yes, ConsaInsights offers customized market report data for the Covid19 Impact On Critical Care Device industry tailored to specific client needs, including targeted analysis and in-depth market insights.
What deliverables can I expect from this Covid19 Impact On Critical Care Device market research project?
From the Covid19 Impact On Critical Care Device market research project, clients can expect comprehensive reports including market segmentation, forecasts, regional analysis, competitive landscape, and strategic recommendations tailored to business objectives.
What are the market trends of Covid19 Impact On Critical Care Device?
Current market trends in the Covid19 Impact On Critical Care Device sector include the rise of telemedicine, innovation in digital health technologies, and an increasing focus on patient-centered care strategies to enhance service delivery.