Critical Care Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: critical-care-diagnostics
Critical Care Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This market report provides a comprehensive analysis of the Critical Care Diagnostics sector, including current trends, market size, growth forecasts from 2023 to 2033, and detailed insights by region and product segment.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
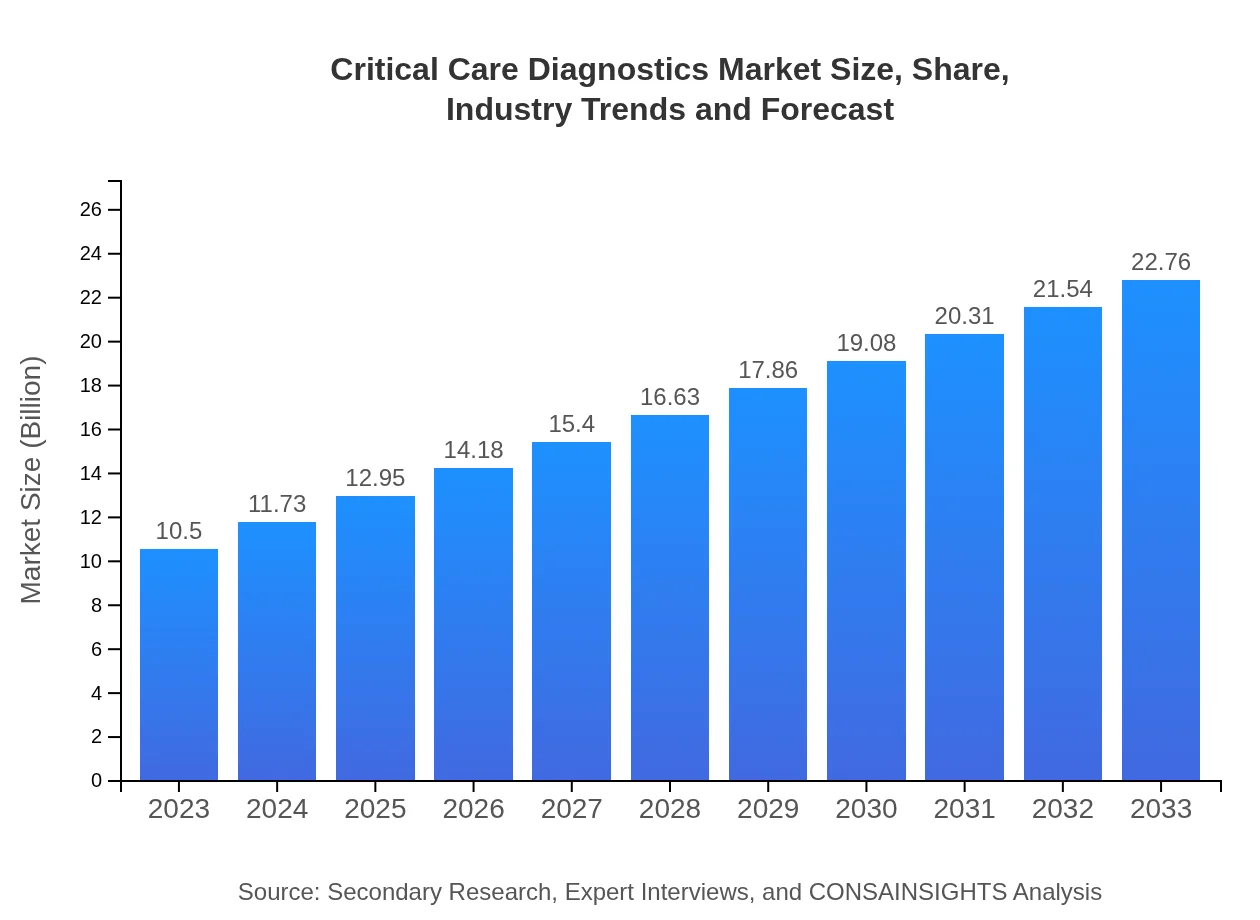
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $22.76 Billion |
| Top Companies | Abbott Laboratories, Siemens Healthineers, Roche Diagnostics, Philips Healthcare |
| Last Modified Date | 31 January 2026 |
Critical Care Diagnostics Market Overview
Customize Critical Care Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Critical Care Diagnostics market size, growth, and forecasts.
- ✔ Understand Critical Care Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Critical Care Diagnostics
What is the Market Size & CAGR of Critical Care Diagnostics market in 2023 and 2033?
Critical Care Diagnostics Industry Analysis
Critical Care Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Critical Care Diagnostics Market Analysis Report by Region
Europe Critical Care Diagnostics Market Report:
The European market for critical care diagnostics is expected to expand from $3.01 billion in 2023 to $6.53 billion by 2033, showcasing a CAGR of 8.05%. Major contributions will arise from significant investments in R&D for healthcare technologies in countries like Germany, France, and the UK. Additionally, increasing incidences of chronic diseases and advancements in diagnostic imaging techniques are expected to enhance market growth.Asia Pacific Critical Care Diagnostics Market Report:
The Asia Pacific region presents significant growth potential, driven by an increase in healthcare investments and a rise in the geriatric population. From a market value of $1.94 billion in 2023, it is projected to reach $4.21 billion by 2033, showcasing a CAGR of 7.92%. The demand for advanced diagnostic systems in both urban and rural healthcare facilities is rising, bolstered by government initiatives to improve healthcare infrastructure.North America Critical Care Diagnostics Market Report:
North America remains the largest market for critical care diagnostics, forecasted to grow from $3.98 billion in 2023 to $8.63 billion by 2033, reflecting a CAGR of 8.12%. The rise in the prevalence of lifestyle-related health issues, advancements in healthcare technology, and a well-established healthcare infrastructure contribute to this robust growth. Moreover, the COVID-19 pandemic has increased demand for rapid diagnostics, propelling further investment in this market.South America Critical Care Diagnostics Market Report:
In South America, the critical care diagnostics market is poised for growth, expanding from $0.79 billion in 2023 to $1.72 billion by 2033, recording a CAGR of 8.28%. This growth is driven by increasing healthcare expenditures and an upward trend in chronic disease rates. Brazil and Argentina are expected to lead the market due to their large populations and continually evolving healthcare systems.Middle East & Africa Critical Care Diagnostics Market Report:
The market in the Middle East and Africa is comparatively smaller, projected to grow from $0.77 billion in 2023 to $1.68 billion by 2033, with a CAGR of 8.30%. The market is driven by rising healthcare budgets and a growing demand for healthcare services. Increased adoption of advanced diagnostics technology in urban healthcare sectors further fuels this market's growth in countries such as South Africa and UAE.Tell us your focus area and get a customized research report.
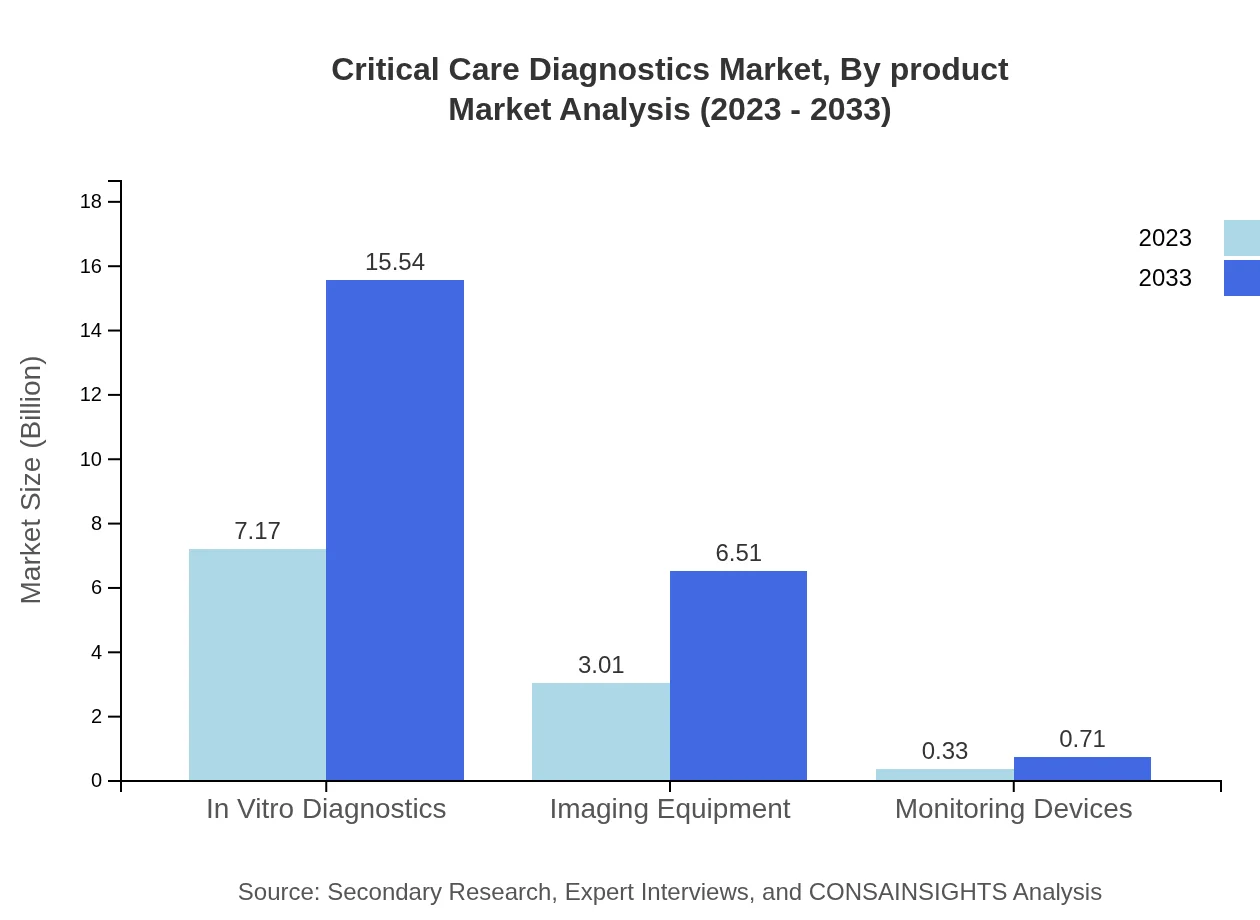
Critical Care Diagnostics Market Analysis By Product
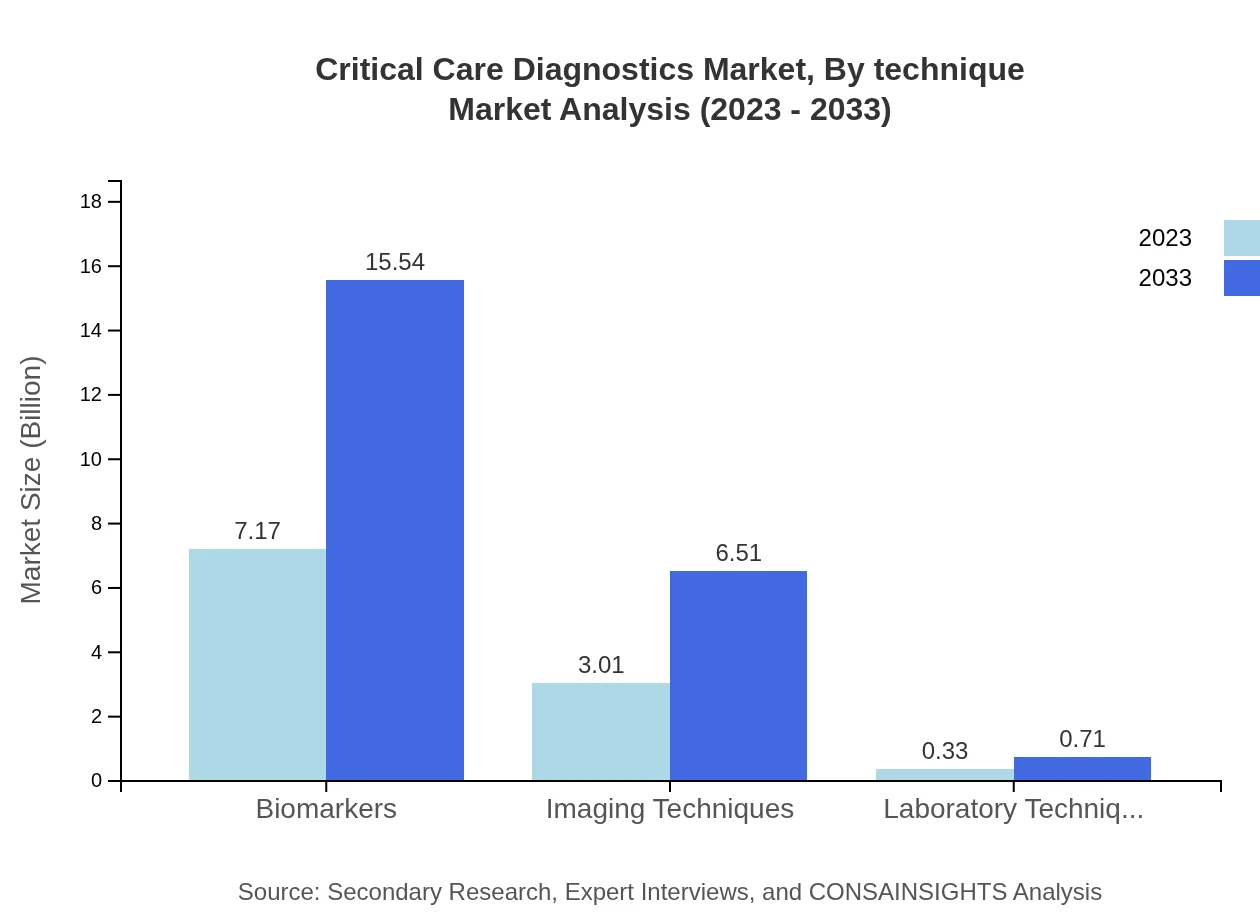
The in vitro diagnostics segment leads the market, valued at $7.17 billion in 2023 and projected to grow to $15.54 billion by 2033, maintaining a substantial market share of 68.26%. Imaging equipment follows with a market value of $3.01 billion in 2023, expected to reach $6.51 billion by 2033, with a share of 28.62%. Monitoring devices currently constitute a smaller segment, growing from $0.33 billion in 2023 to $0.71 billion by 2033, holding 3.12% of market share.
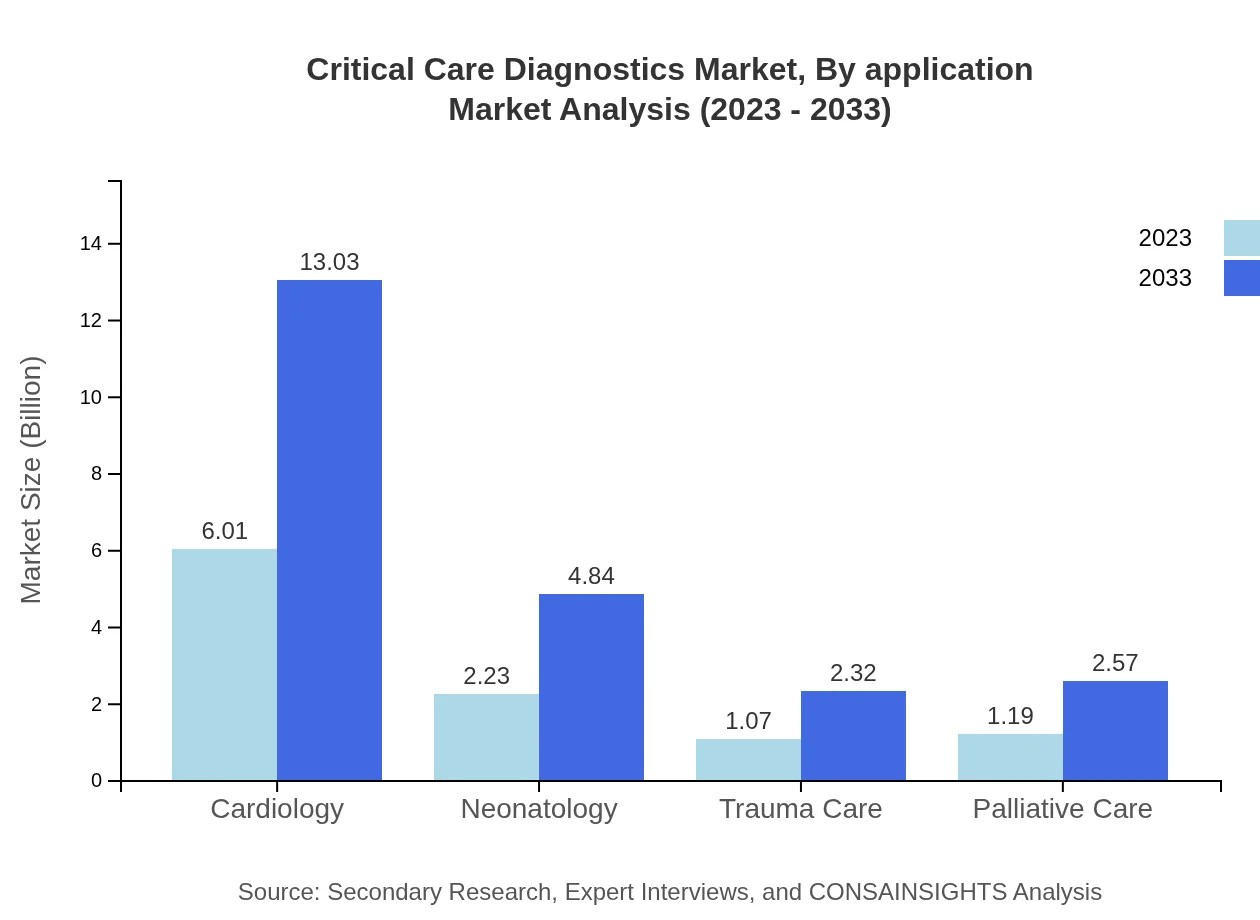
Critical Care Diagnostics Market Analysis By Application
In terms of applications, cardiology remains the leading segment, with a market size of $6.01 billion in 2023 and projected growth to $13.03 billion by 2033, holding a share of 57.23%. Neonatology is also significant, growing from $2.23 billion in 2023 to $4.84 billion in 2033, representing a share of 21.28%. Other applications, such as palliative care and trauma care, are experiencing growth driven by advancements in diagnostic tools.
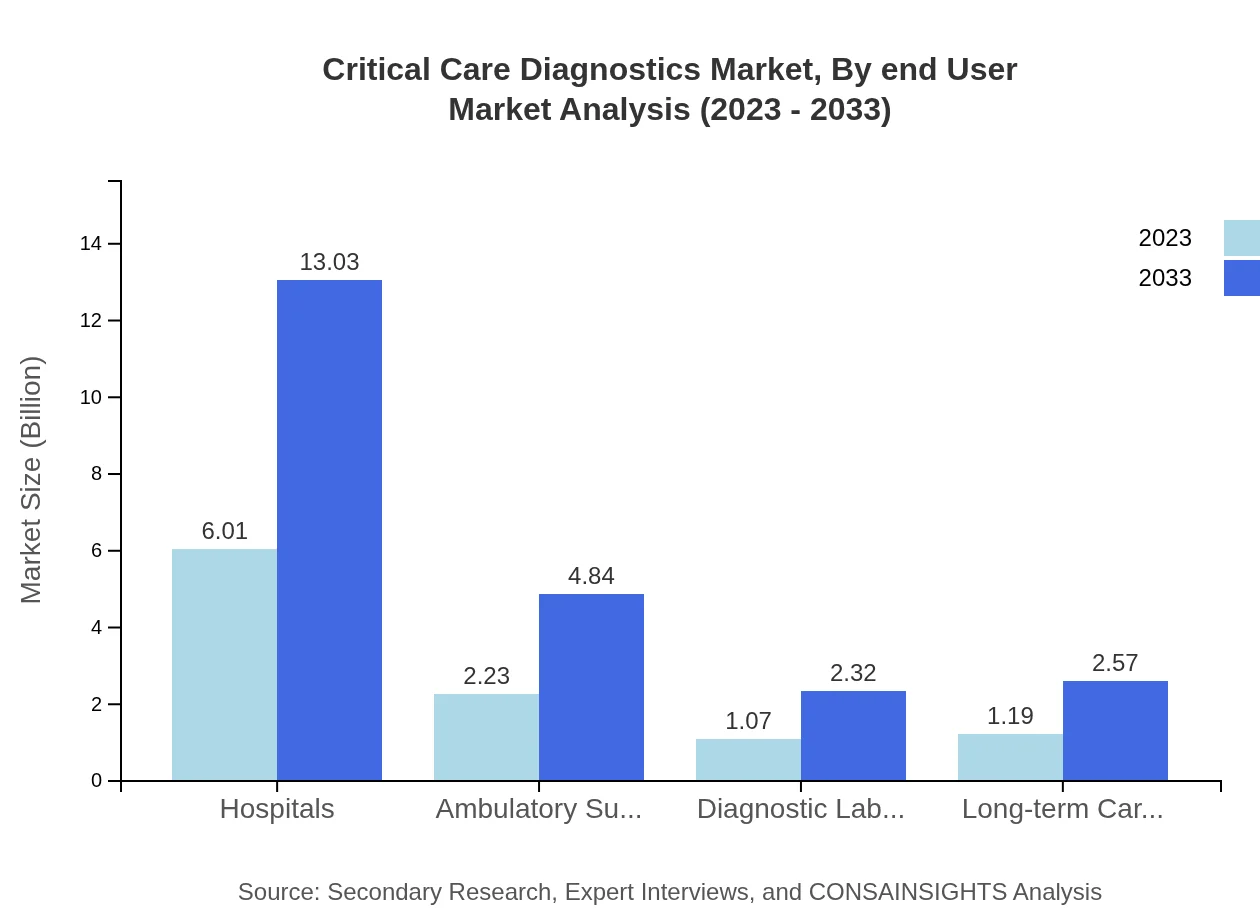
Critical Care Diagnostics Market Analysis By End User
Hospitals dominate the end-user segment with a value of $6.01 billion in 2023, forecasted to escalate to $13.03 billion by 2033, accounting for 57.23% market share. Ambulatory surgical centers are also notable, expected to grow from $2.23 billion in 2023 to $4.84 billion in 2033 with a 21.28% share. The increasing number of outpatient procedures and surgeries is driving this segment forward.
Critical Care Diagnostics Market Analysis By Technique
The laboratory techniques segment is experiencing steady growth, while imaging and biomarker analyses are expected to gain traction. Techniques focusing on quick diagnostics are in high demand, emphasizing the need for rapid and highly accurate test results in critical care settings.
Critical Care Diagnostics Market Analysis By Distribution Channel
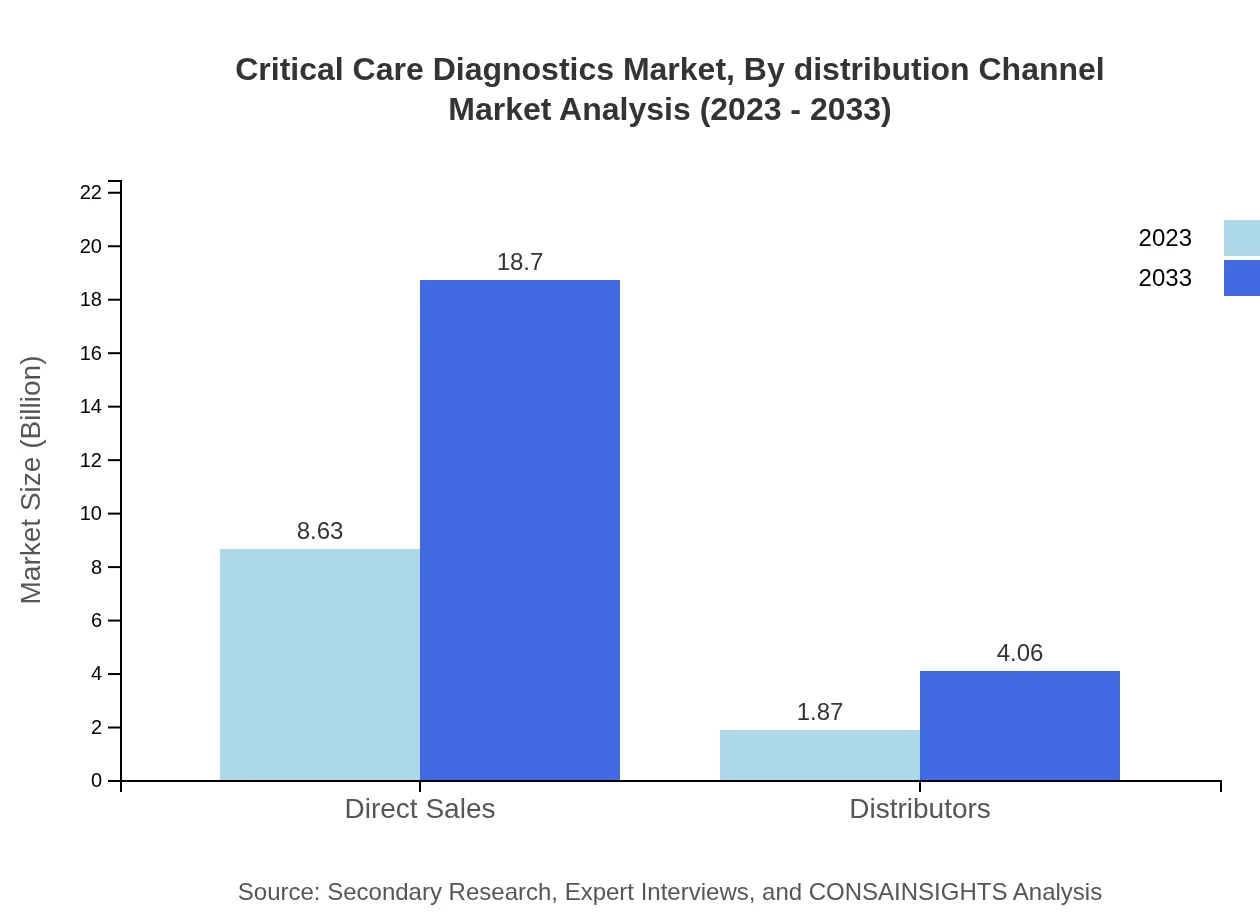
Direct sales account for a significant market share at $8.63 billion in 2023, with expectations to reach $18.70 billion by 2033, comprising 82.15% of the market share. Distributors support this market, growing from $1.87 billion in 2023 to $4.06 billion by 2033, capturing 17.85% of the market share.
Critical Care Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Critical Care Diagnostics Industry
Abbott Laboratories:
Abbott is a global leader in diagnostics and medical devices, specializing in developing innovative solutions for healthcare improvement, particularly in critical care settings.Siemens Healthineers:
Siemens Healthineers is known for its advanced imaging and diagnostic technologies, offering a comprehensive range of products for critical case management.Roche Diagnostics:
Roche Diagnostics focuses on in vitro diagnostics and is at the forefront of providing advanced tests and services across various healthcare systems.Philips Healthcare:
Philips Healthcare specializes in patient monitoring and imaging solutions, contributing significantly to the evolution of diagnostic capabilities in critical care.We're grateful to work with incredible clients.









FAQs
What is the market size of critical Care Diagnostics?
The critical care diagnostics market is valued at $10.5 billion in 2023, with a projected CAGR of 7.8% from 2023 to 2033, indicating notable growth in demand for diagnostic tools in critical care settings.
What are the key market players or companies in this critical Care Diagnostics industry?
Key players in the critical care diagnostics industry include major healthcare companies that specialize in diagnostic devices and technologies aimed at critical care. These organizations are vital for innovation and growth in the market.
What are the primary factors driving the growth in the critical Care diagnostics industry?
The growth of the critical care diagnostics industry is driven by increasing incidences of chronic diseases, advancements in diagnostic technologies, and a rising demand for rapid diagnostic tests in critical care scenarios.
Which region is the fastest Growing in the critical Care diagnostics?
The fastest-growing region in the critical care diagnostics market is North America, projected to increase from $3.98 billion in 2023 to $8.63 billion by 2033, reflecting strong demand and market expansion.
Does ConsaInsights provide customized market report data for the critical Care diagnostics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the critical care diagnostics sector, allowing clients to gain insights that are most relevant to their operations and strategies.
What deliverables can I expect from this critical Care diagnostics market research project?
From the critical care diagnostics market research project, you can expect comprehensive reports including market size, growth forecasts, regional analysis, and competitive landscape assessments tailored to your specific interests.
What are the market trends of critical Care diagnostics?
Current market trends include the shift towards home healthcare diagnostics, increased integration of AI in diagnostic tools, and a growing focus on preventive care, reflecting evolving patient needs and technology advancements.