Cystic Fibrosis Cf Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: cystic-fibrosis-cf-therapeutics
Cystic Fibrosis Cf Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cystic Fibrosis Cf Therapeutics market, covering trends, segmentation, and regional insights from 2023 to 2033. It aims to deliver valuable data and forecasts to stakeholders in understanding market dynamics and growth prospects.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
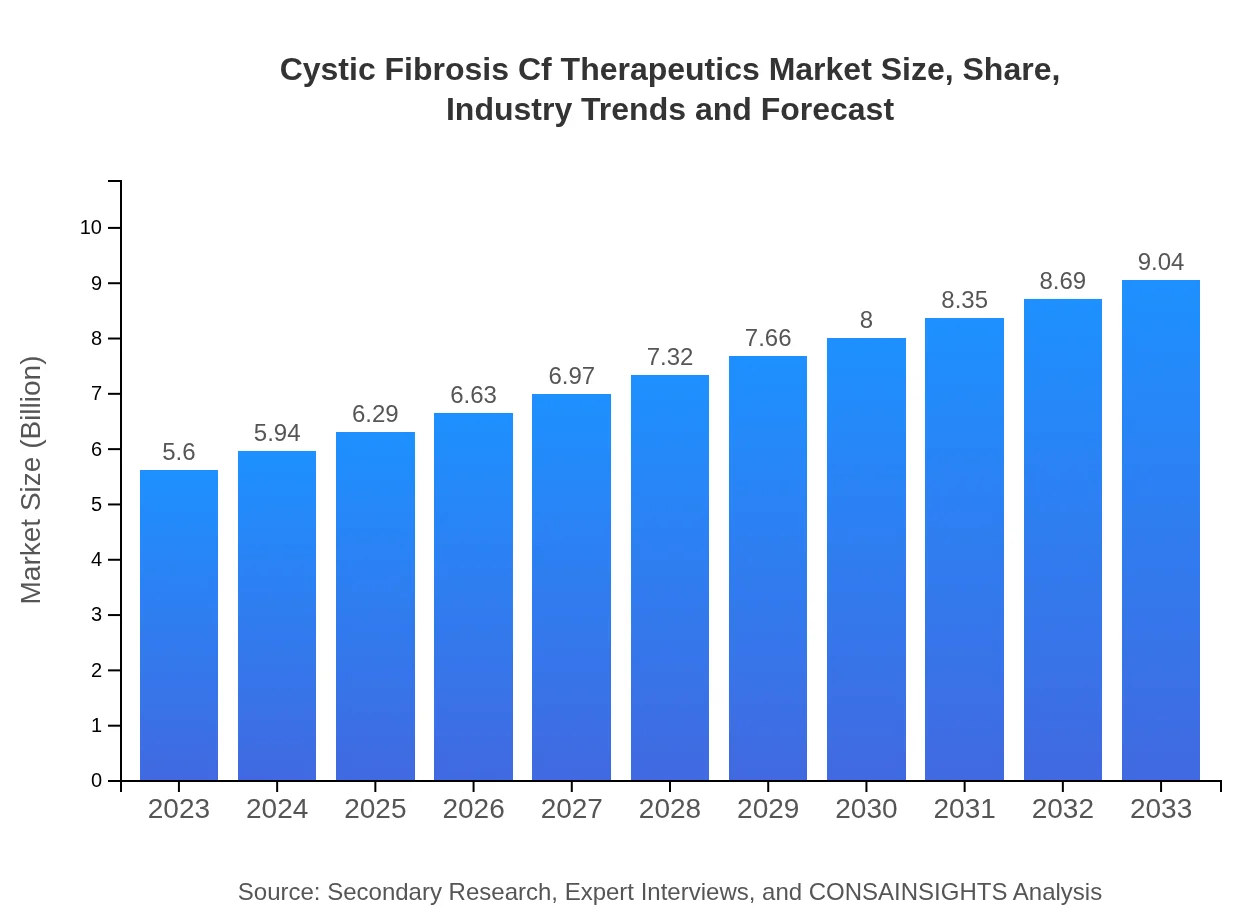
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 4.8% |
| 2033 Market Size | $9.04 Billion |
| Top Companies | Vertex Pharmaceuticals, AbbVie, Gilead Sciences, Teva Pharmaceutical Industries, Novartis |
| Last Modified Date | 31 January 2026 |
Cystic Fibrosis Cf Therapeutics Market Overview
Customize Cystic Fibrosis Cf Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Cystic Fibrosis Cf Therapeutics market size, growth, and forecasts.
- ✔ Understand Cystic Fibrosis Cf Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cystic Fibrosis Cf Therapeutics
What is the Market Size & CAGR of Cystic Fibrosis Cf Therapeutics market in 2023?
Cystic Fibrosis Cf Therapeutics Industry Analysis
Cystic Fibrosis Cf Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cystic Fibrosis Cf Therapeutics Market Analysis Report by Region
Europe Cystic Fibrosis Cf Therapeutics Market Report:
The European market is projected to grow from $1.55 billion in 2023 to $2.50 billion by 2033. Regulatory support and initiatives for orphan drugs enhance the appeal of CF therapeutics, encouraging innovations and improved patient access across various European countries.Asia Pacific Cystic Fibrosis Cf Therapeutics Market Report:
In the Asia Pacific region, the market for Cystic Fibrosis Cf Therapeutics is projected to grow from $1.12 billion in 2023 to $1.81 billion by 2033. This growth is supported by collaborations between local manufacturers and global entities to ensure access to cutting-edge treatments, as well as increasing awareness about the disease.North America Cystic Fibrosis Cf Therapeutics Market Report:
North America dominates the Cystic Fibrosis market, with market size expected to rise from $1.89 billion in 2023 to $3.05 billion by 2033. The expansion is attributed to advanced healthcare infrastructure, high disposable incomes, and significant investments in research for CF treatments, alongside favorable reimbursement policies.South America Cystic Fibrosis Cf Therapeutics Market Report:
The South American market for Cystic Fibrosis Cf Therapeutics, while smaller, is anticipated to expand from $0.26 billion in 2023 to $0.42 billion in 2033. Challenges such as limited healthcare budgets and accessibility are being addressed through increased investments and partnerships aimed at enhancing care delivery.Middle East & Africa Cystic Fibrosis Cf Therapeutics Market Report:
The Middle East and Africa market is expected to increase from $0.78 billion in 2023 to $1.26 billion by 2033, driven by rising healthcare investments and growing awareness of cystic fibrosis, despite facing challenges like infrastructure deficits and access issues.Tell us your focus area and get a customized research report.
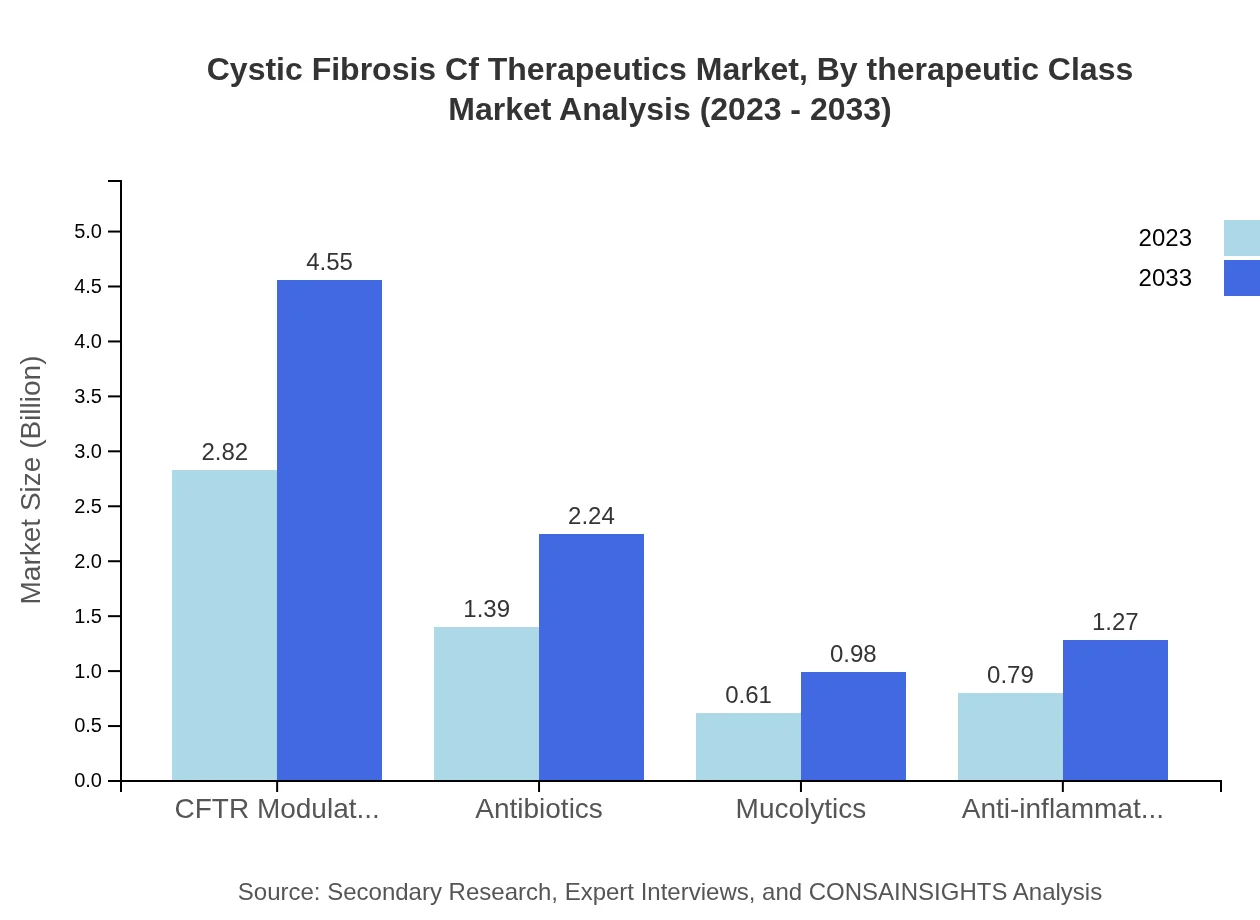
Cystic Fibrosis Cf Therapeutics Market Analysis By Therapeutic Class
Key therapeutic classes include CFTR modulators, antibiotics, mucolytics, and anti-inflammatory drugs. CFTR modulators represent a significant section of the market, projected to account for more than 50% of the market share by 2033 as they address the root cause of the disease. Antibiotics continue to play a critical role in managing infections, holding around 24.84% market share in 2023.
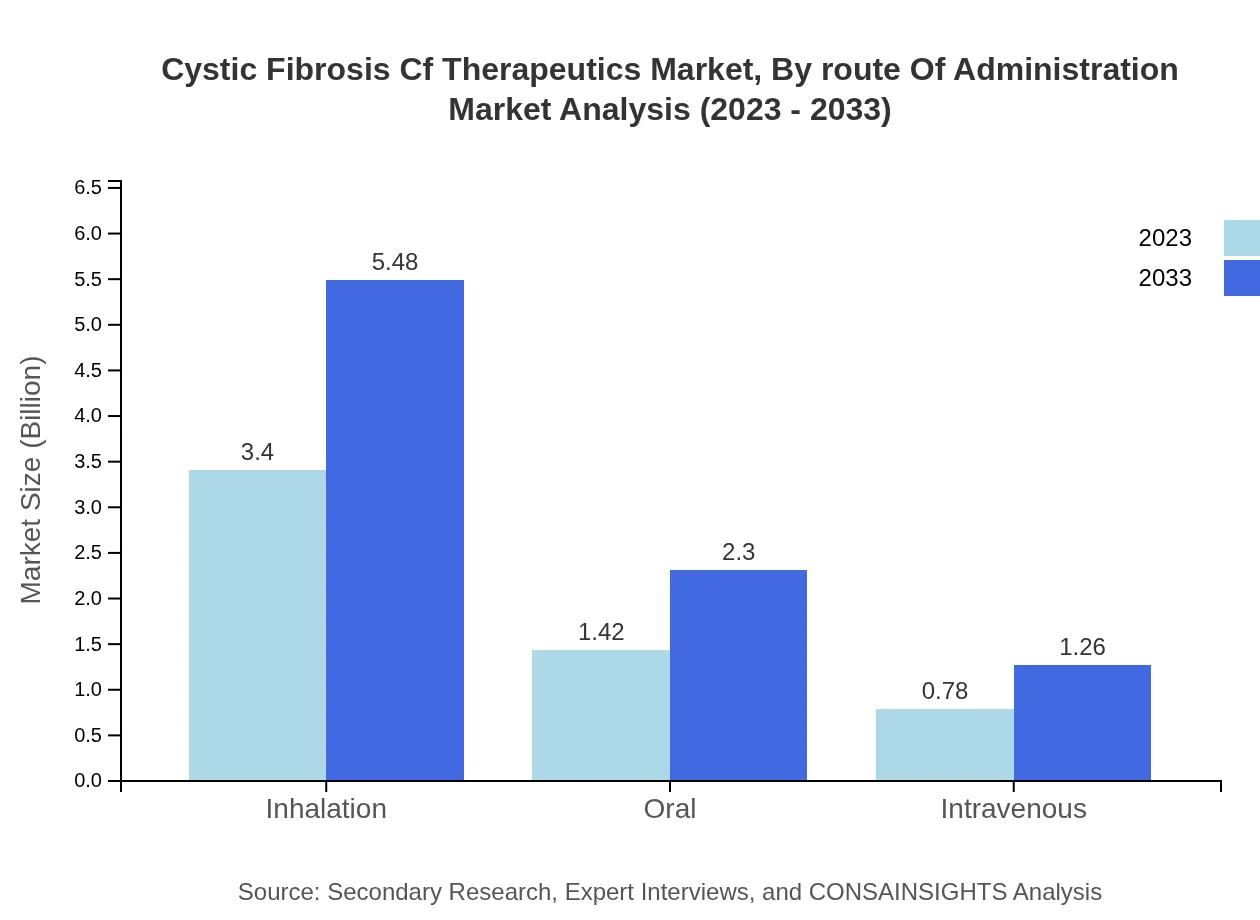
Cystic Fibrosis Cf Therapeutics Market Analysis By Route Of Administration
The route of administration segment highlights inhalation therapies as a predominant choice (60.68% share in 2023), reflecting the effectiveness of delivering medications directly to the lungs. Oral and intravenous routes also retain specialized niches, focusing on patient preferences and treatment protocols.
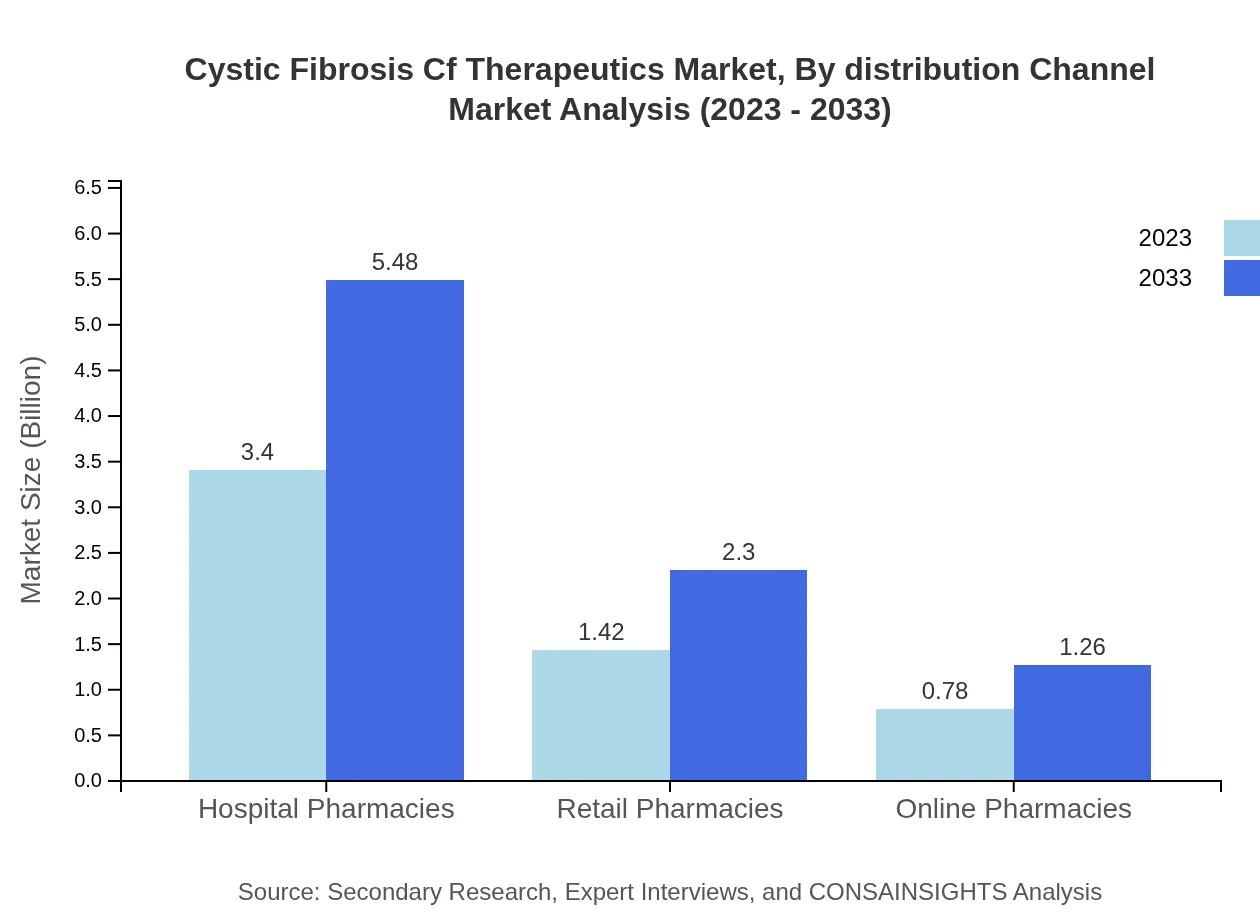
Cystic Fibrosis Cf Therapeutics Market Analysis By Distribution Channel
Hospital pharmacies dominate the distribution channel for CF therapeutics, accounting for over 60% market share, reflecting the critical role of healthcare professionals in managing treatment protocols. Retail and online pharmacies are gaining ground due to increasing patient mobility and preferences for accessible medication.
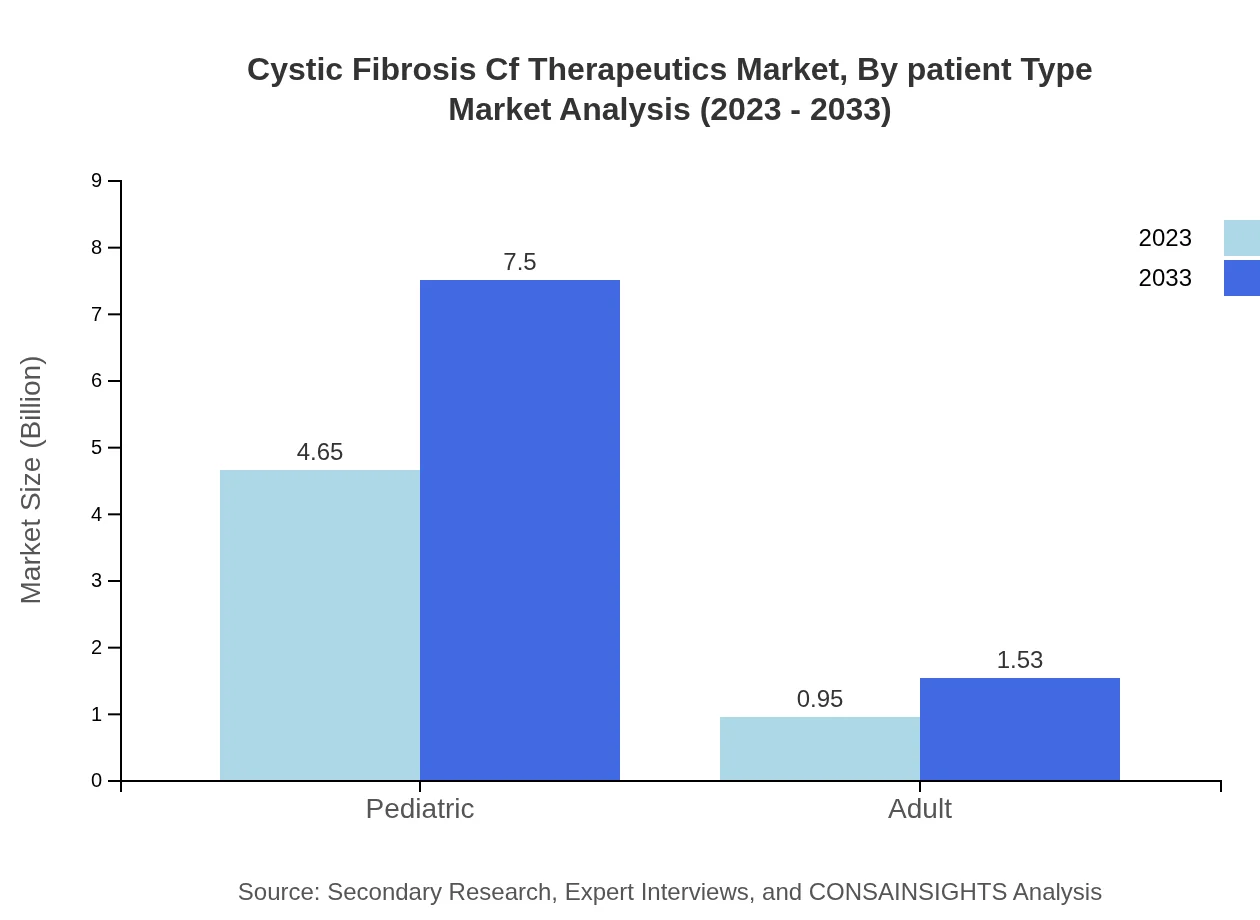
Cystic Fibrosis Cf Therapeutics Market Analysis By Patient Type
The majority share of the market is attributed to pediatric patients (83.06% share), underlining the importance of early diagnosis and ongoing treatment in managing Cystic Fibrosis effectively. Adult patient needs, while smaller in comparison, are important for designing comprehensive treatment strategies.
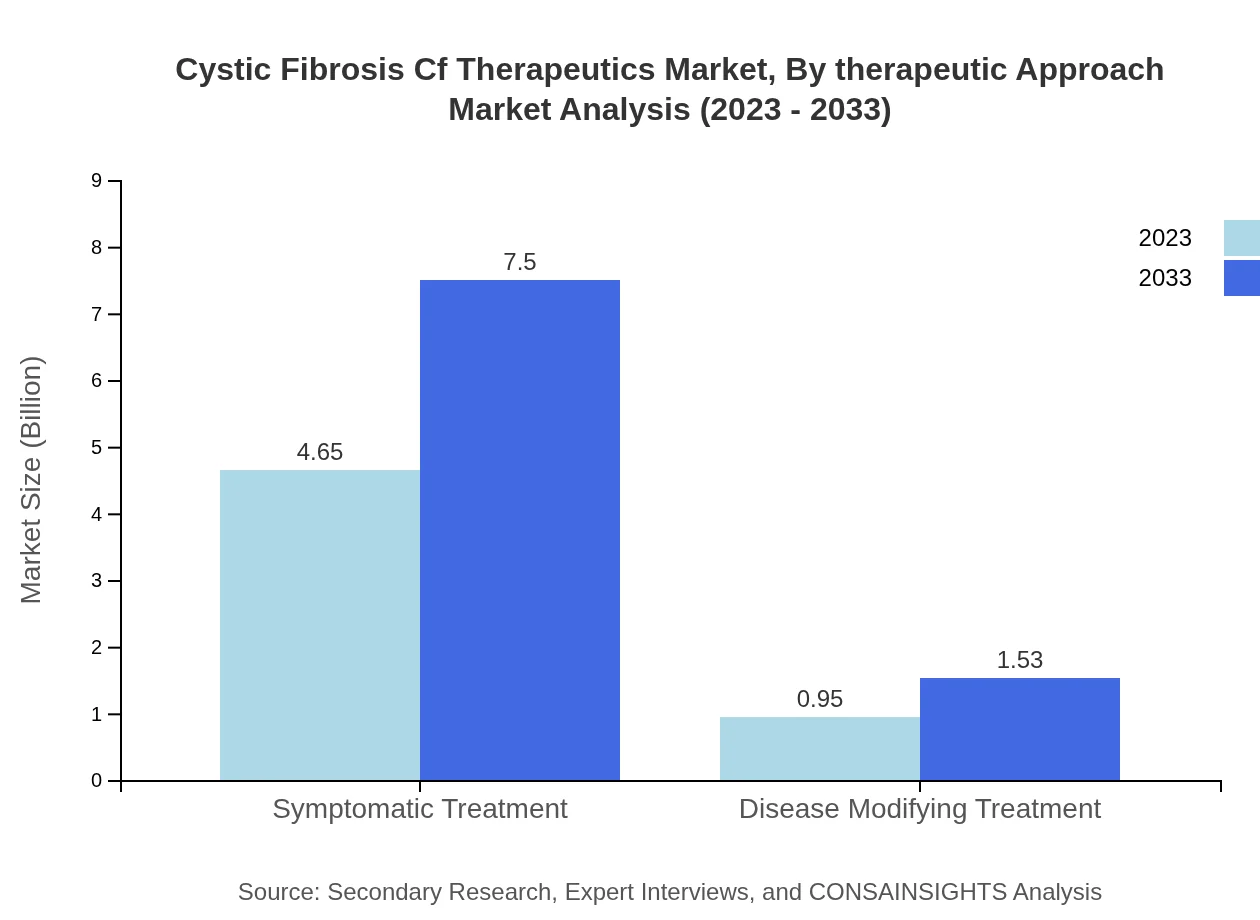
Cystic Fibrosis Cf Therapeutics Market Analysis By Therapeutic Approach
With a focus on symptomatic and disease-modifying treatment approaches, the market reflects a dual strategy toward managing Cystic Fibrosis. Symptomatic treatments currently hold a substantial share due to immediate patient needs, while disease-modifying treatments are gaining traction as research progresses.
Cystic Fibrosis Cf Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cystic Fibrosis Cf Therapeutics Industry
Vertex Pharmaceuticals:
A leader in CF therapies, Vertex is known for its breakthrough CFTR modulators, including Kalydeco, Orkambi, and Trikafta, significantly enhancing treatment efficacy.AbbVie:
AbbVie offers a range of therapies for CF and has invested heavily in research and collaborations to develop innovative treatment strategies, enhancing patient care.Gilead Sciences:
Gilead is prominent for its infectious disease therapies, providing antibiotics crucial for CF patients to manage lung infections effectively.Teva Pharmaceutical Industries:
Teva plays a critical role in generics and specialty medications, contributing to expanding treatment accessibility for CF patients across various regions.Novartis:
Novartis focuses on innovative therapeutic solutions for CF, bolstering market presence through strategic partnerships and pipeline development.We're grateful to work with incredible clients.









FAQs
What is the market size of cystic Fibrosis Cf Therapeutics?
The global Cystic Fibrosis (CF) therapeutics market is valued at approximately $5.6 billion in 2023, with a projected compound annual growth rate (CAGR) of 4.8%, indicating robust growth prospects through 2033.
What are the key market players or companies in the cystic Fibrosis Cf Therapeutics industry?
Key players in the CF therapeutics market include major pharmaceutical companies such as Vertex Pharmaceuticals, AbbVie, and Genentech. These companies are at the forefront of developing innovative therapies aimed at improving the quality of life for CF patients.
What are the primary factors driving the growth in the cystic Fibrosis Cf Therapeutics industry?
Growth in the CF therapeutics market is driven by factors such as increasing prevalence of cystic fibrosis, advancements in treatment options, the introduction of personalized medicine, and growing healthcare investments in research and development.
Which region is the fastest Growing in the cystic Fibrosis Cf Therapeutics?
North America is the fastest-growing region in the CF therapeutics market, projected to increase from $1.89 billion in 2023 to $3.05 billion by 2033, reflecting significant advancements in treatment availability and healthcare infrastructure.
Does ConsaInsights provide customized market report data for the cystic Fibrosis Cf Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to meet specific client needs in the cystic fibrosis therapeutics sector. Our analysis includes comprehensive insights into market dynamics, trends, and forecasts.
What deliverables can I expect from this cystic Fibrosis Cf Therapeutics market research project?
Clients can expect a detailed report encompassing market size, growth forecasts, competitive landscape analysis, segment specifics, regional insights, and key trends in the CF therapeutics market, all supported by actionable insights.
What are the market trends of cystic Fibrosis Cf Therapeutics?
Current trends in the CF therapeutics market include a shift towards personalized therapies, increasing use of CFTR modulators, growing emphasis on combination therapies, and a rise in patient-centric care approaches.