Cytokine Based Therapies And Inhibitors Market Report
Published Date: 31 January 2026 | Report Code: cytokine-based-therapies-and-inhibitors
Cytokine Based Therapies And Inhibitors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cytokine Based Therapies and Inhibitors market, focusing on insights and data from 2023 to 2033, including market dynamics, trends, segmentation, regional analysis, and forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
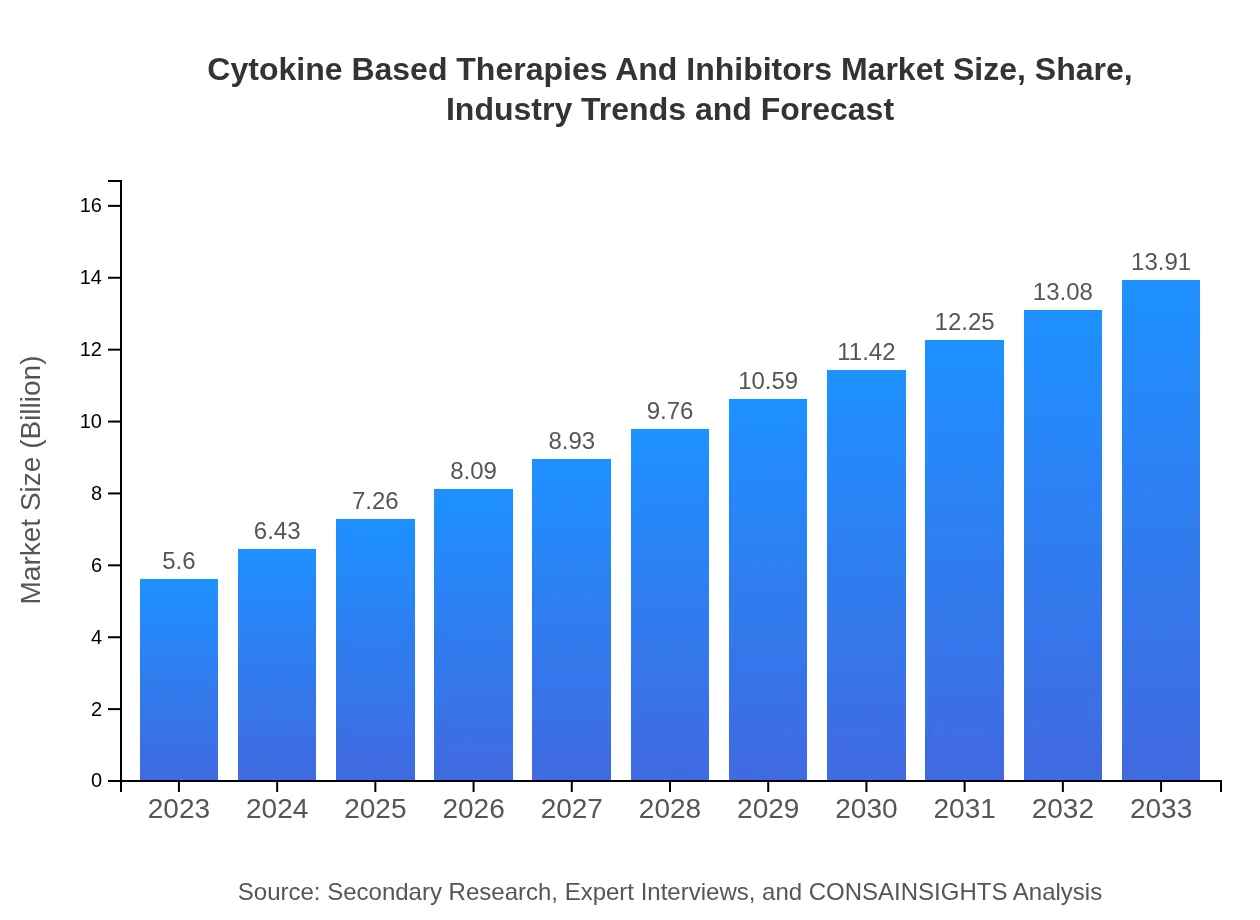
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $13.91 Billion |
| Top Companies | AbbVie, Roche, Amgen, Sanofi, Novartis |
| Last Modified Date | 31 January 2026 |
Cytokine Based Therapies And Inhibitors Market Overview
Customize Cytokine Based Therapies And Inhibitors Market Report market research report
- ✔ Get in-depth analysis of Cytokine Based Therapies And Inhibitors market size, growth, and forecasts.
- ✔ Understand Cytokine Based Therapies And Inhibitors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cytokine Based Therapies And Inhibitors
What is the Market Size & CAGR of Cytokine Based Therapies And Inhibitors market in 2023?
Cytokine Based Therapies And Inhibitors Industry Analysis
Cytokine Based Therapies And Inhibitors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cytokine Based Therapies And Inhibitors Market Analysis Report by Region
Europe Cytokine Based Therapies And Inhibitors Market Report:
The European market is poised to rise from $1.68 billion in 2023 to $4.18 billion in 2033. The European region benefits from strong regulatory frameworks and a high prevalence of autoimmune disorders. Healthcare systems in Germany and France are notably supportive of therapeutic advancements.Asia Pacific Cytokine Based Therapies And Inhibitors Market Report:
The Asia-Pacific market is set to grow from approximately $1.06 billion in 2023 to $2.63 billion by 2033, driven by increasing healthcare expenditure, growing patient populations, and rising investments in biopharmaceutical innovations. Countries like China and India are at the forefront, enhancing R&D initiatives and facilitating market entry for global players.North America Cytokine Based Therapies And Inhibitors Market Report:
North America remains the largest market, with a valuation jumping from $2.04 billion in 2023 to $5.07 billion by 2033. The increase is propelled by robust investments in healthcare and biotechnology, along with advancements in technology and infrastructure that support drug innovation. The U.S. is a leader in both research and regulatory approvals.South America Cytokine Based Therapies And Inhibitors Market Report:
In South America, the market is expected to expand from $0.10 billion in 2023 to $0.25 billion in 2033. Regional market growth is supported by increasing access to healthcare services and rising incidences of chronic diseases, with Brazil and Argentina being the key contributors to market dynamics.Middle East & Africa Cytokine Based Therapies And Inhibitors Market Report:
In the Middle East and Africa, the market is foreseen expanding from $0.71 billion in 2023 to $1.78 billion by 2033, driven by an increase in healthcare spending, improved access to advanced therapies, and rising awareness of cytokine therapies in managing diseases.Tell us your focus area and get a customized research report.
Cytokine Based Therapies And Inhibitors Market Analysis By Product
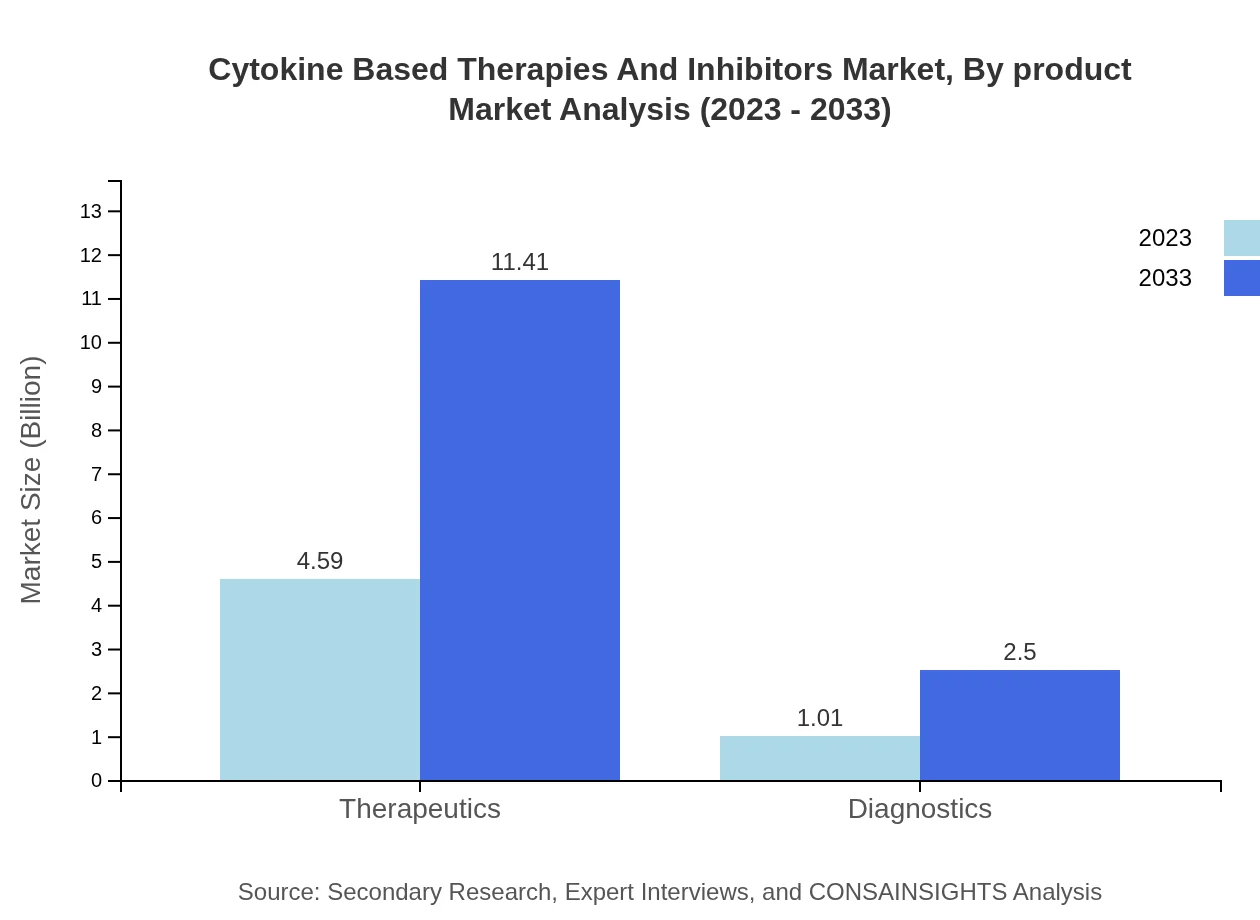
The Cytokine-Based Therapies and Inhibitors market exhibits significant growth potential in therapeutics, which represents approximately 82% of the total market. The therapeutic segment is projected to grow from $4.59 billion in 2023 to $11.41 billion by 2033. In contrast, diagnostic products, while smaller, are expected to grow from $1.01 billion to $2.50 billion, marking a growing interest in precision medicine.
Cytokine Based Therapies And Inhibitors Market Analysis By Therapeutic Area
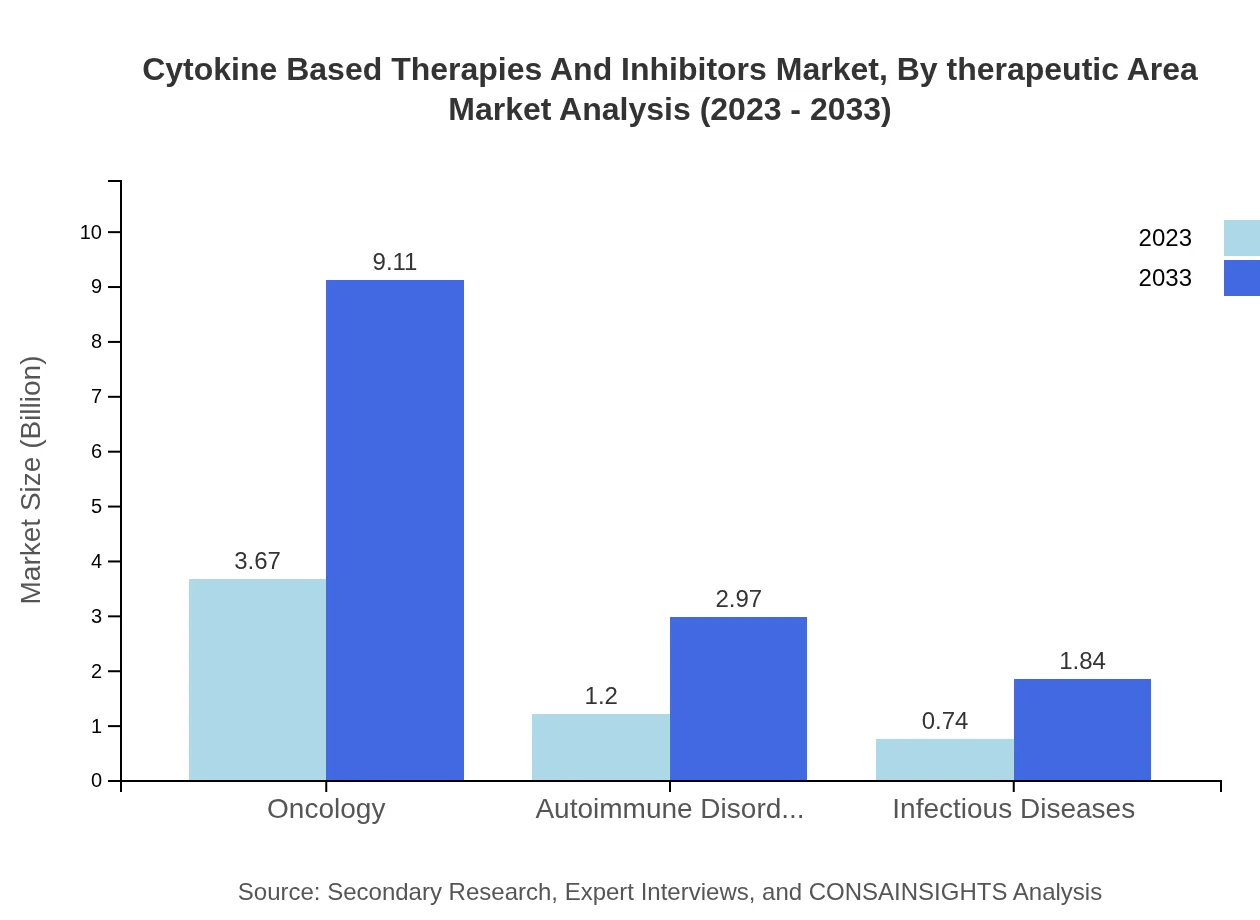
Oncology remains the dominant therapeutic area, capturing a considerable market share, with size growing from $3.67 billion in 2023 to $9.11 billion by 2033. This segment witnesses significant investment as novel therapies for cancer are urgently needed. Autoimmune disorders and infectious diseases also hold promising positions, with respective market sizes expected to grow to $2.97 billion and $1.84 billion by the projected year.
Cytokine Based Therapies And Inhibitors Market Analysis By Route Of Administration
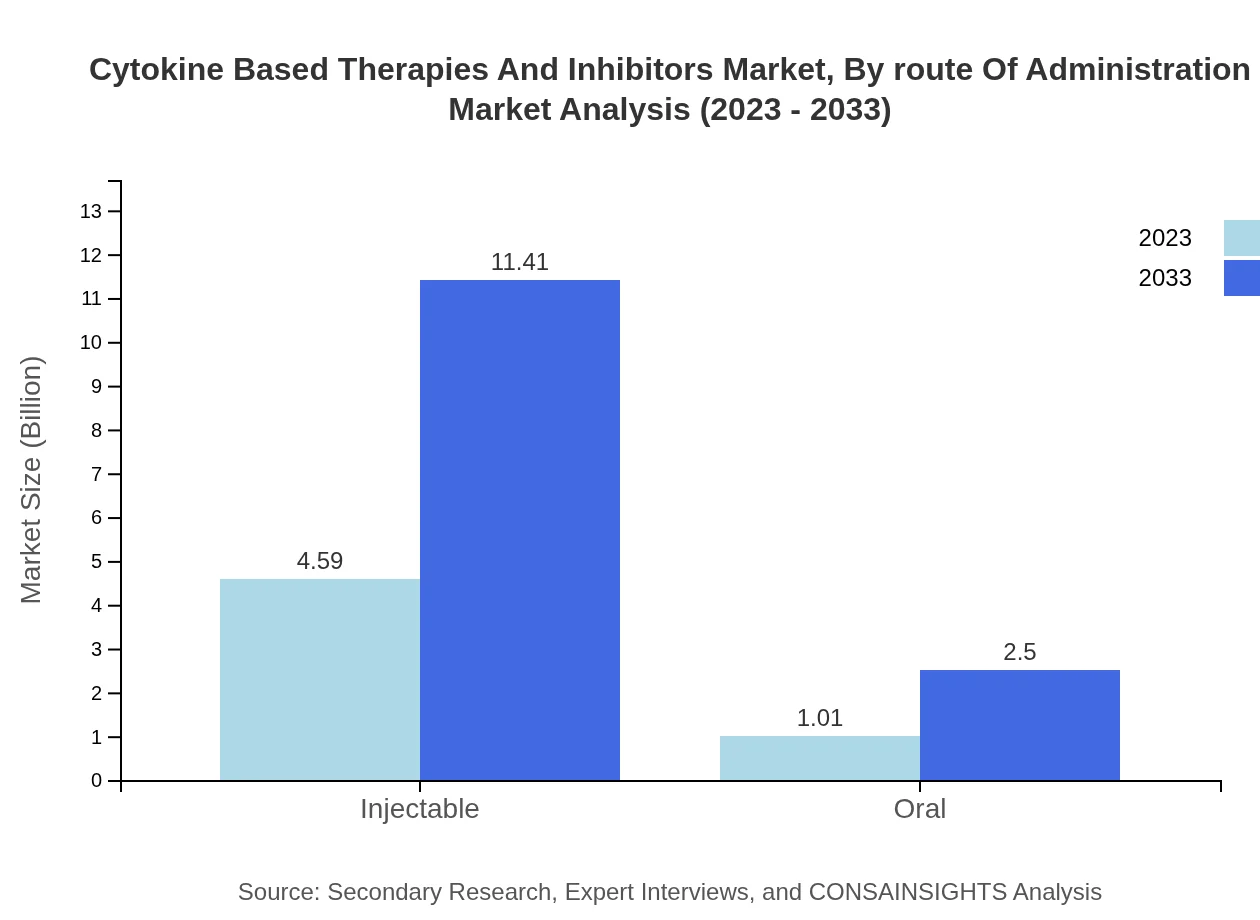
Injectable forms dominate the route of administration segment, accounting for approximately 82% of market share. Expected to increase from $4.59 billion in 2023 to $11.41 billion by 2033, injectables are favored for their direct impact and faster efficacy. Oral formulations are becoming more widely accepted, growing from $1.01 billion to $2.50 billion, as companies strive to enhance convenience for patients.
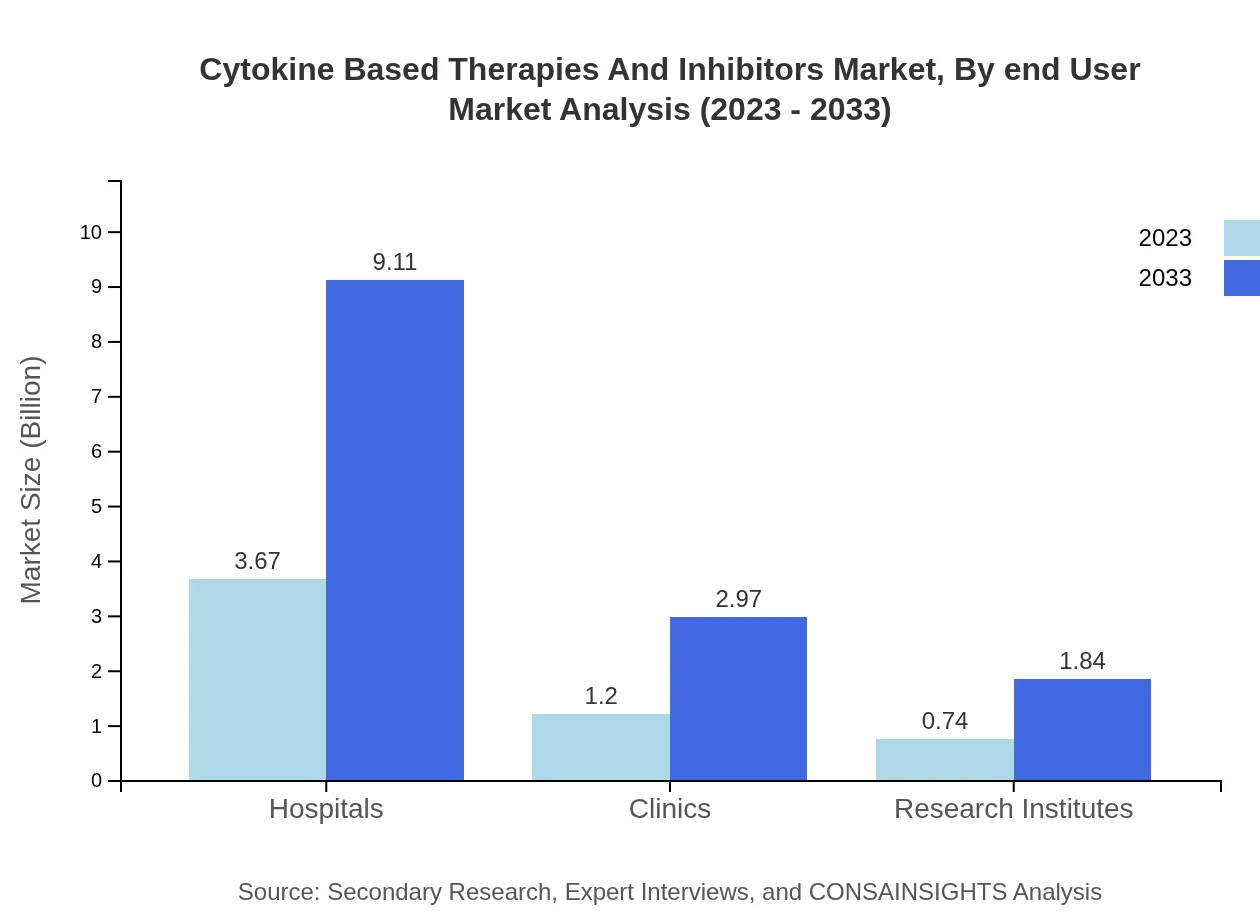
Cytokine Based Therapies And Inhibitors Market Analysis By End User
Hospitals represent the primary end-user segment, expected to grow from $3.67 billion in 2023 to $9.11 billion by 2033, holding approximately 65% of the market share. Clinics and research institutes also constitute significant segments, relying on the deployment of cytokine therapies in specialized patient care and clinical studies.
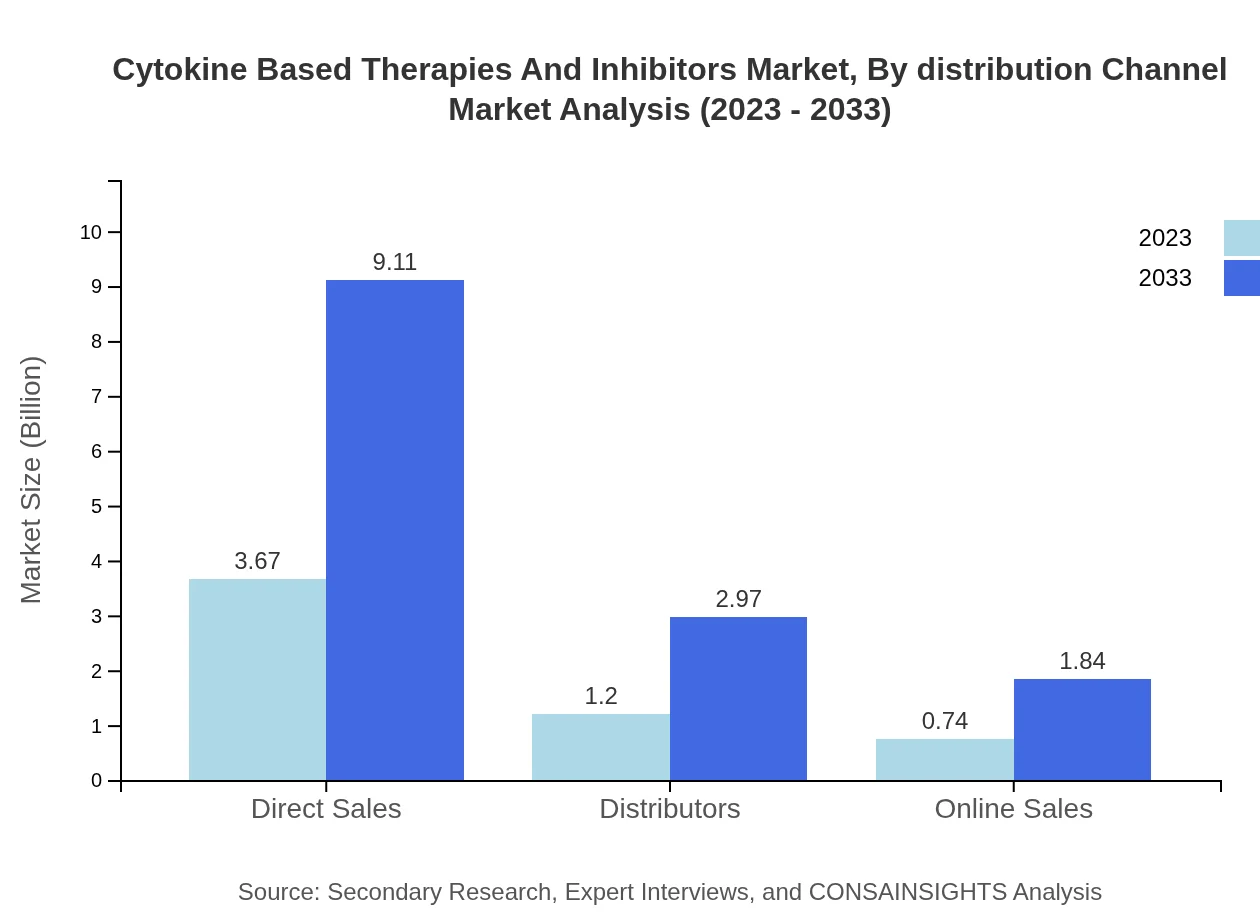
Cytokine Based Therapies And Inhibitors Market Analysis By Distribution Channel
Direct sales currently dominate this market, representing about 65% market share. This segment is expanding significantly as manufacturers seek to foster close relationships with healthcare providers. However, online sales and distributor channels are rapidly gaining traction, aiming to provide broader access to therapies, expected to grow from $0.74 billion to $1.84 billion.
Cytokine Based Therapies And Inhibitors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cytokine Based Therapies And Inhibitors Industry
AbbVie:
AbbVie is an established leader in the cytokine market, well-known for its immunology pipeline including innovative biologics for autoimmune disorders.Roche:
Roche is pivotal in the development of various cytokine therapies and diagnostics, leveraging extensive research capabilities and global partnerships.Amgen:
With a strong focus on oncology and immunotherapy, Amgen contributes significantly to advancements in cytokine-based therapies.Sanofi:
Sanofi is actively developing a range of cytokine-based therapeutics, particularly in the fields of immunology and oncology.Novartis:
Novartis focuses on the research and development of cutting-edge cytokine therapies aimed at cancer and autoimmune diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of cytokine Based therapies and inhibitors?
The global market size for cytokine-based therapies and inhibitors in 2023 is estimated at approximately $5.6 billion, with a projected CAGR of 9.2% from 2023 to 2033, indicating robust growth in this sector.
What are the key market players or companies in the cytokine Based therapies and inhibitors industry?
Key players in the cytokine therapies sector include major pharmaceutical companies such as AbbVie, Amgen, BMS, and Pfizer, focusing on innovations in treatments targeting autoimmune diseases and cancers, among other conditions.
What are the primary factors driving the growth in the cytokine Based therapies and inhibitors industry?
Key factors propelling market growth include increasing incidence of chronic diseases, advancements in biotechnology, and a rise in research and development activities aimed at discovering novel therapies improved by cytokine modulation.
Which region is the fastest Growing in the cytokine Based therapies and inhibitors?
The fastest-growing region for cytokine-based therapies and inhibitors is Europe, with the market projected to increase from $1.68 billion in 2023 to $4.18 billion by 2033, reflecting substantial growth opportunities.
Does ConsaInsights provide customized market report data for the cytokine Based therapies and inhibitors industry?
Yes, ConsaInsights offers customized market report data, tailored to specific industry needs, covering aspects such as market trends, competitive landscape, and growth forecasts, ensuring clients receive relevant insights.
What deliverables can I expect from this cytokine Based therapies and inhibitors market research project?
Deliverables include comprehensive reports featuring market size analysis, trend evaluations, competitive insights, segmented data, and forecasts until 2033, providing a complete overview for strategic decision-making.
What are the market trends of cytokine Based therapies and inhibitors?
Current market trends include a shift towards personalized medicine, growth in monoclonal antibodies, and an emphasis on combination therapies, reflecting an increasing focus on targeted therapies in cytokine innovation.