D Dimer Market Report
Published Date: 31 January 2026 | Report Code: d-dimer
D Dimer Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the D Dimer market, covering insights on market size, industry trends, segment performance, and forecast data from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
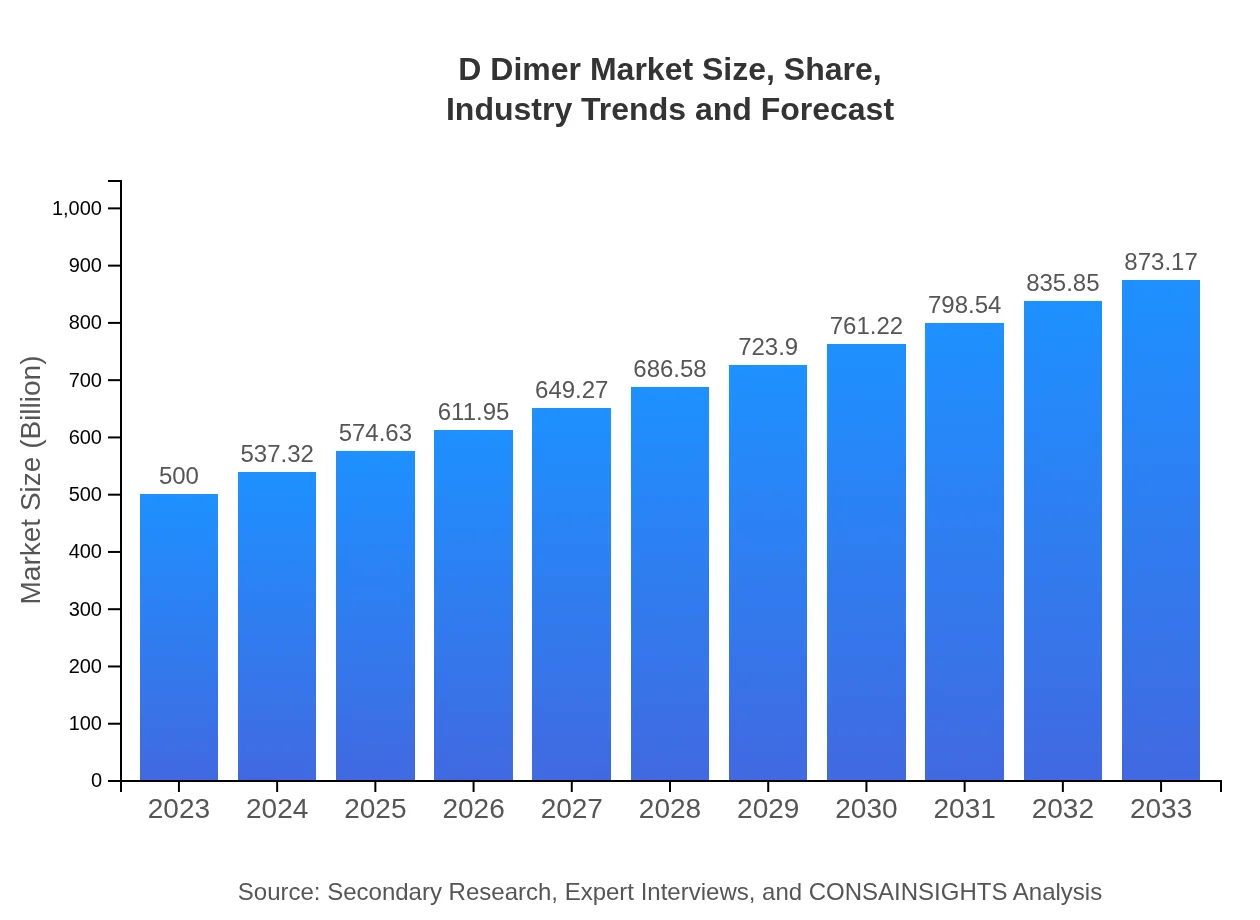
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.6% |
| 2033 Market Size | $873.17 Million |
| Top Companies | Abbott Laboratories, Siemens Healthineers, Roche Diagnostics, Thermo Fisher Scientific, Ortho Clinical Diagnostics |
| Last Modified Date | 31 January 2026 |
D Dimer Market Overview
Customize D Dimer Market Report market research report
- ✔ Get in-depth analysis of D Dimer market size, growth, and forecasts.
- ✔ Understand D Dimer's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in D Dimer
What is the Market Size & CAGR of D Dimer market in 2033?
D Dimer Industry Analysis
D Dimer Market Segmentation and Scope
Tell us your focus area and get a customized research report.
D Dimer Market Analysis Report by Region
Europe D Dimer Market Report:
Europe's D Dimer market is poised for growth, valued at around $166.35 million in 2023 and estimated to reach $290.50 million by 2033. The increasing prevalence of cardiovascular diseases and supportive regulatory frameworks contribute significantly to this growth.Asia Pacific D Dimer Market Report:
In the Asia Pacific region, the D Dimer market was valued at approximately $82.40 million in 2023, with projections to reach $143.90 million by 2033. The growth is attributed to increasing healthcare expenditure, rising incidences of thromboembolic disorders, and advancements in diagnostic technologies.North America D Dimer Market Report:
North America is a leading market for D Dimer tests, with a size of $180.25 million in 2023, projected to rise to $314.78 million by 2033. The region benefits from advanced healthcare infrastructure, high diagnostic test usage, and constant innovation in testing methodologies.South America D Dimer Market Report:
The South American D Dimer market is expected to grow from $44.35 million in 2023 to $77.45 million by 2033. Factors such as improving healthcare infrastructure, awareness of thrombotic conditions, and increased testing capabilities are driving market expansion in this region.Middle East & Africa D Dimer Market Report:
In the Middle East and Africa, the D Dimer market was valued at $26.65 million in 2023, with expectations to grow to $46.54 million by 2033. The region is experiencing improvements in healthcare access and increasing awareness regarding diagnostic testing, promoting market development.Tell us your focus area and get a customized research report.
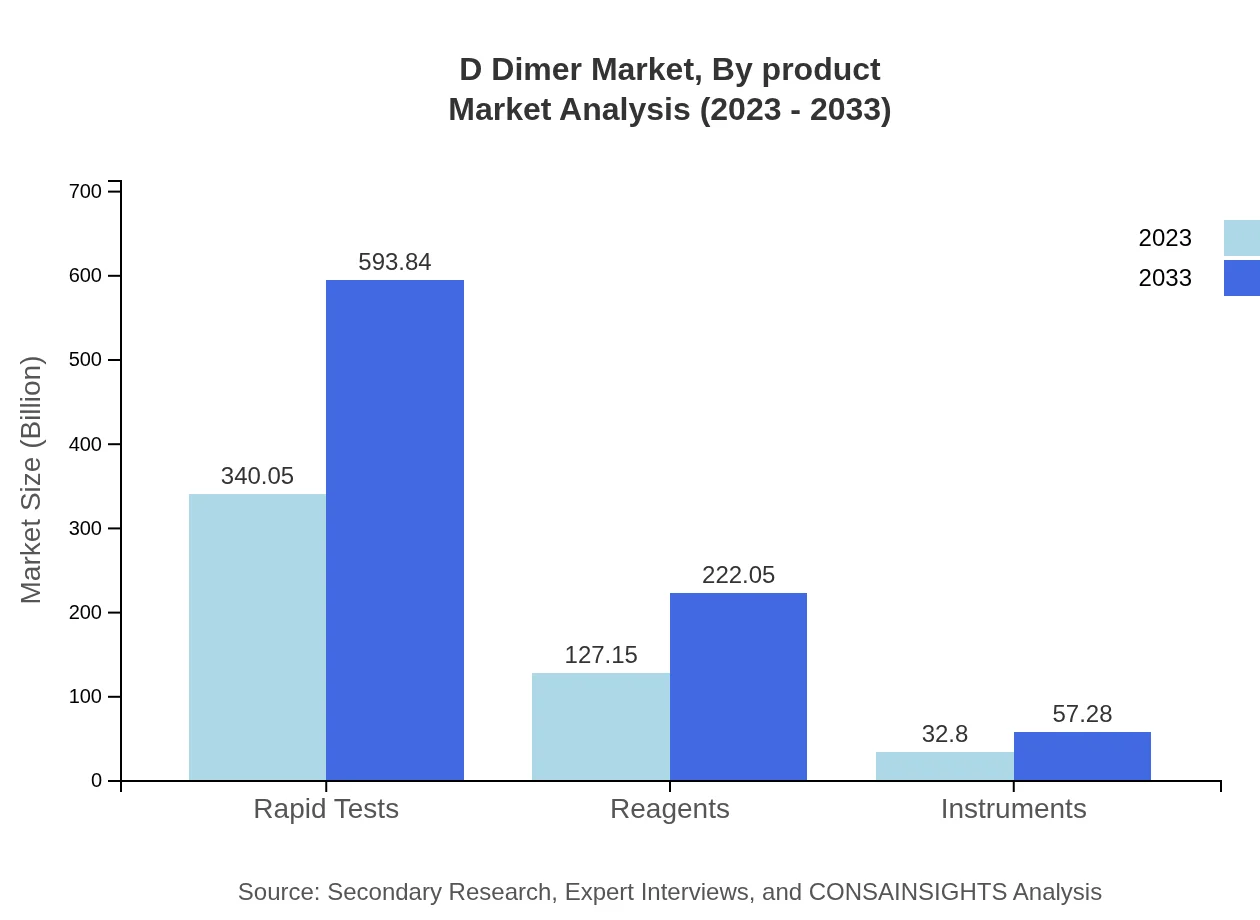
D Dimer Market Analysis By Product
The D Dimer market by product includes Rapid Tests, Reagents, and Instruments. Rapid Tests represent the largest segment, with a market value of $340.05 million in 2023 and projected to reach $593.84 million by 2033. Reagents and Instruments follow, comprising essential components of D Dimer testing processes, with Reagents at $127.15 million (2023) and expected to grow to $222.05 million by 2033.
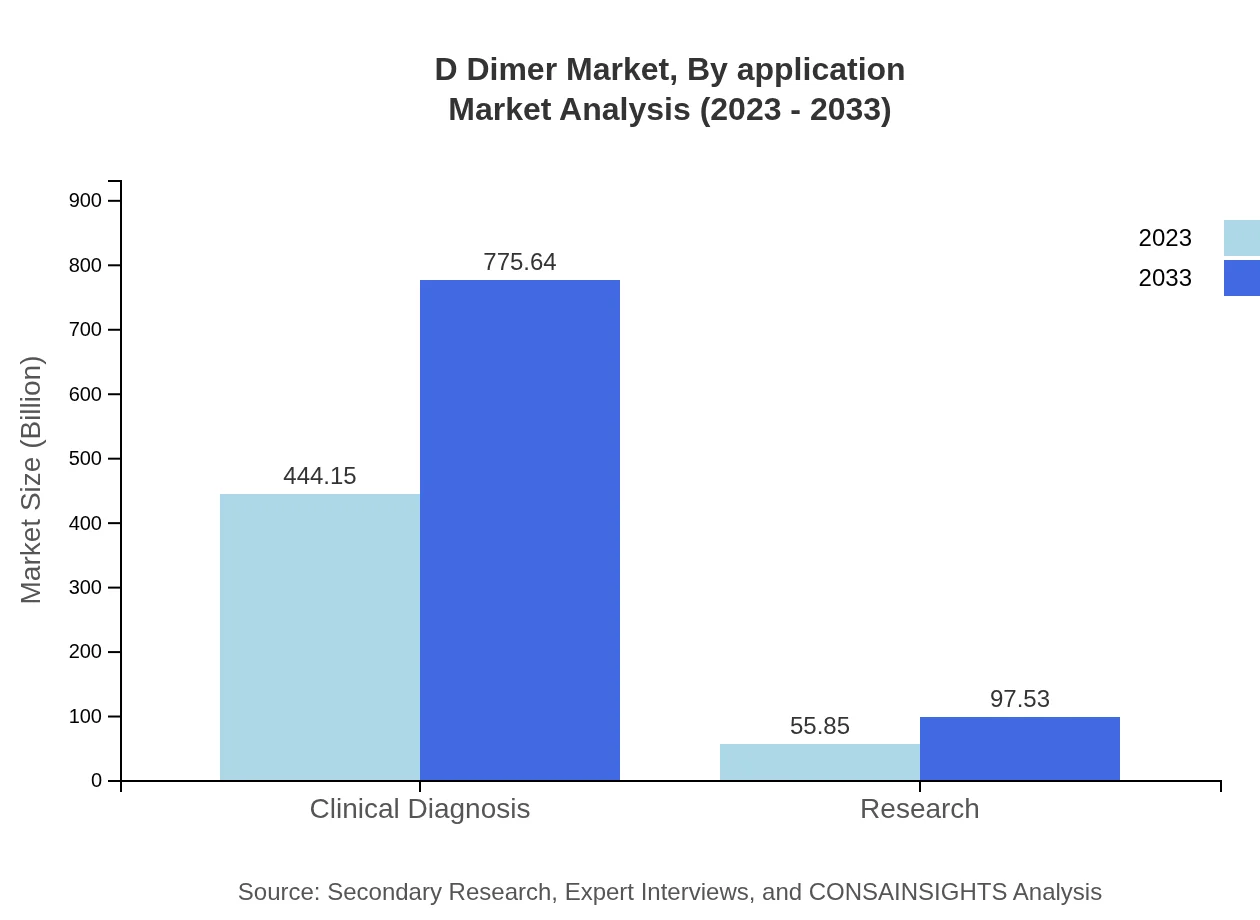
D Dimer Market Analysis By Application
In terms of application, Clinical Diagnosis leads the segment, contributing $444.15 million in 2023 and forecasted to reach $775.64 million by 2033. Research applications also play a significant role, with a market size of $55.85 million in 2023, growing to $97.53 million by 2033.
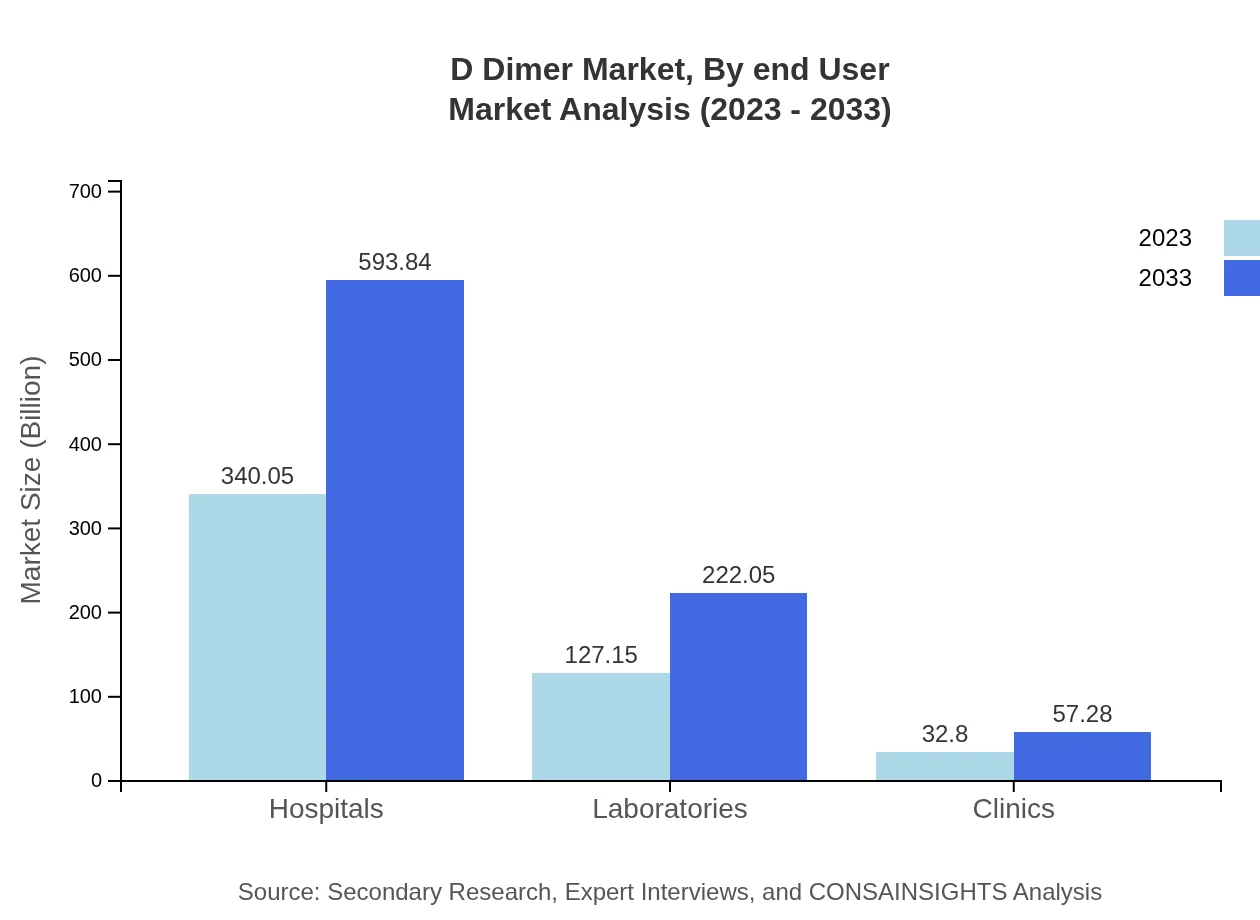
D Dimer Market Analysis By End User
The key end-users of D Dimer tests are Hospitals, Laboratories, and Clinics. Hospitals take a dominant share, with a market size of $340.05 million in 2023 expected to expand to $593.84 million by 2033. Laboratories also show strong growth potential, with values of $127.15 million in 2023 and rising to $222.05 million by 2033.
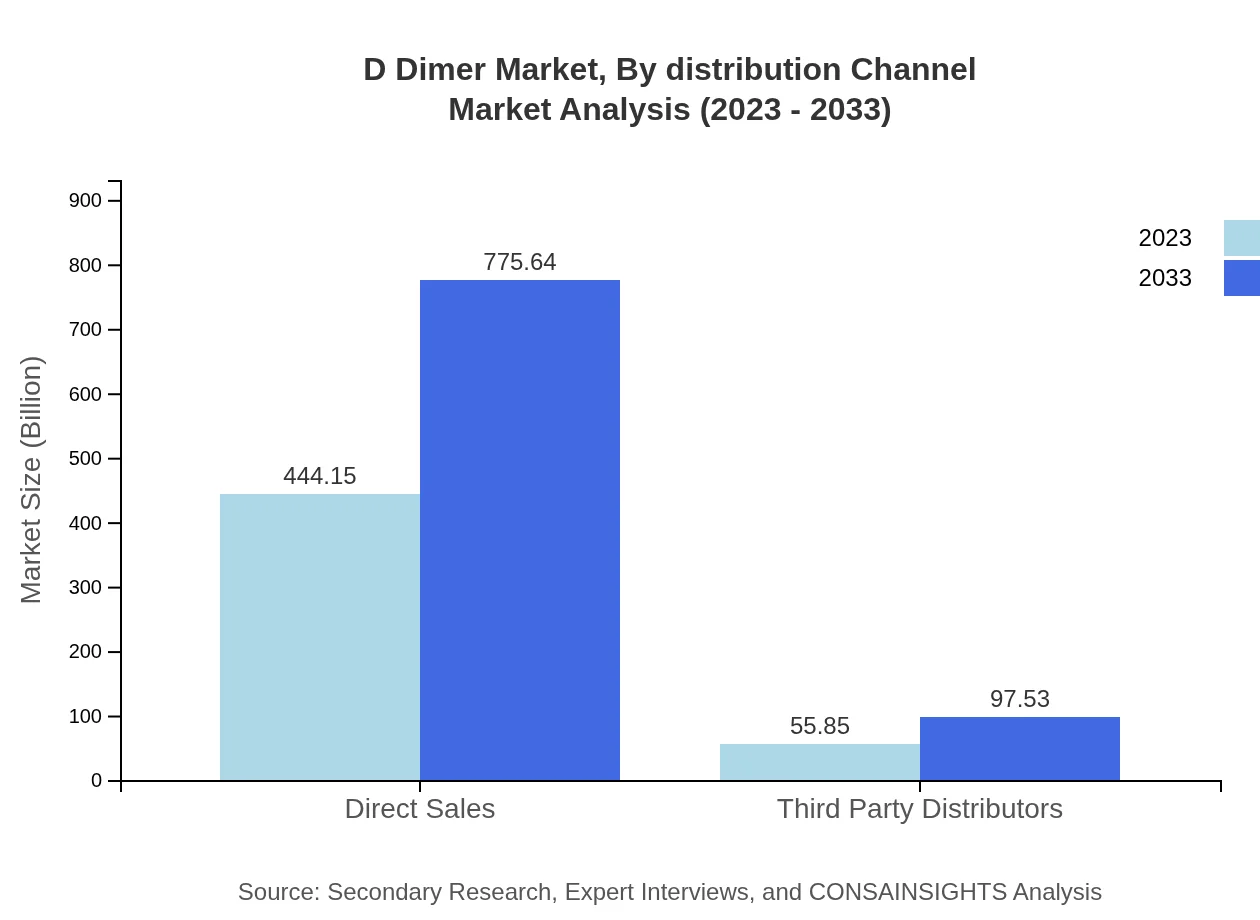
D Dimer Market Analysis By Distribution Channel
Distribution channels for D Dimer products include Direct Sales and Third Party Distributors. Direct Sales are leading with a market size of $444.15 million in 2023, continuing to $775.64 million by 2033. Third Party Distributors also contribute, albeit at a smaller scale, with estimates moving from $55.85 million (2023) to $97.53 million (2033).
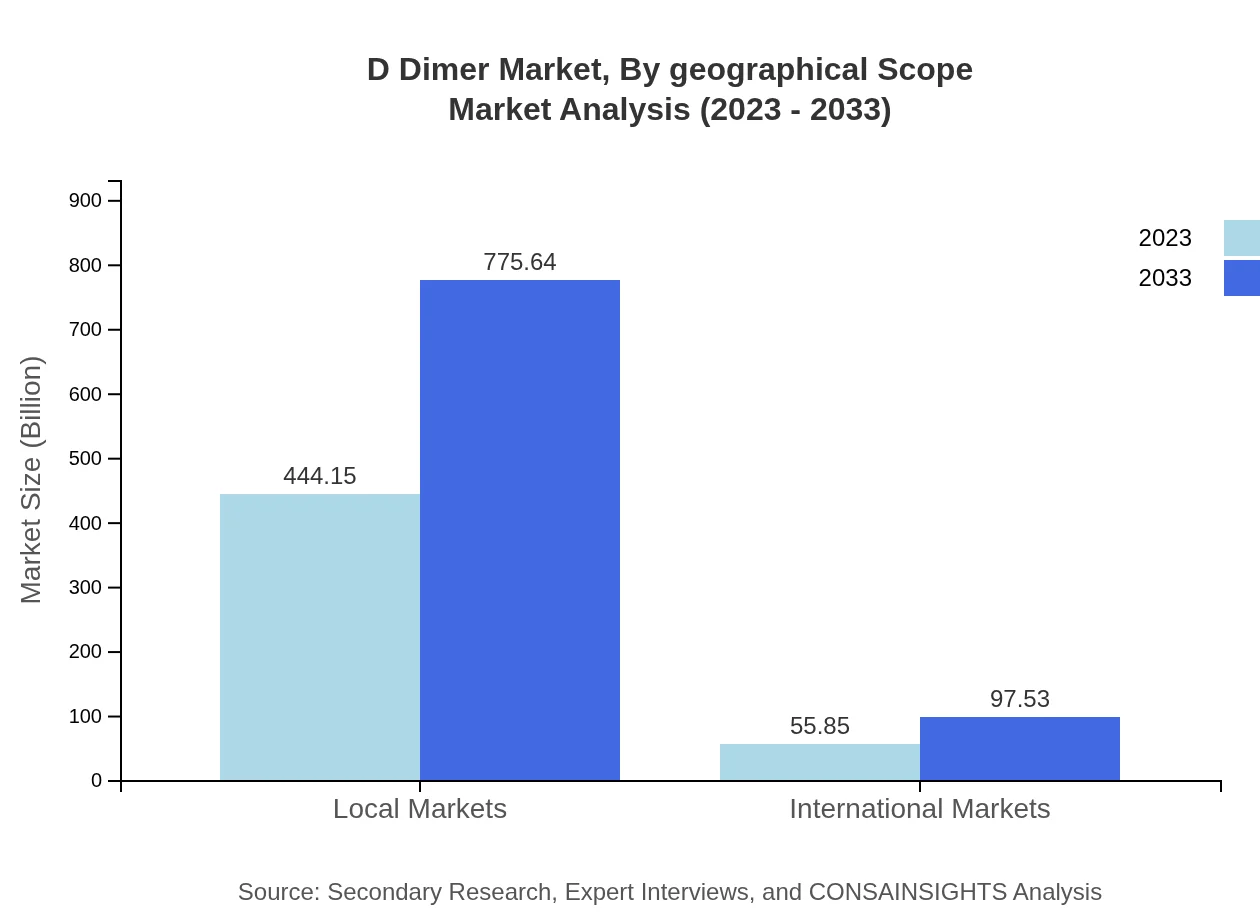
D Dimer Market Analysis By Geographical Scope
Geographical scope covers local and international markets, with local markets comprising $444.15 million in 2023, expected to grow to $775.64 million by 2033. International markets show a smaller share, moving from $55.85 million in 2023 to $97.53 million by 2033.
D Dimer Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in D Dimer Industry
Abbott Laboratories:
Abbott Laboratories is a leading global healthcare company that develops and markets innovative diagnostic tests, including D Dimer assays, significantly improving patient outcomes in thromboembolic conditions.Siemens Healthineers:
Siemens Healthineers specializes in medical diagnostics and imaging systems, contributing significantly to the D Dimer market with high-quality and efficient testing solutions.Roche Diagnostics:
Roche Diagnostics is a prominent player in the diagnostics space, offering cutting-edge D Dimer tests that enhance clinical decision-making and patient care.Thermo Fisher Scientific:
Thermo Fisher Scientific specializes in providing high-quality diagnostic products, including D Dimer testing solutions that meet industry standards.Ortho Clinical Diagnostics:
Ortho Clinical Diagnostics delivers innovative testing solutions in hemoglobin and D Dimer assays, playing a crucial role in the diagnostic landscape.We're grateful to work with incredible clients.









FAQs
What is the market size of D Dimer?
The global D-dimer market is currently valued at approximately $500 million with an expected compound annual growth rate (CAGR) of 5.6% from 2023 to 2033.
What are the key market players or companies in the D Dimer industry?
Key players in the D-dimer industry include major diagnostic companies such as Roche, Siemens Healthineers, Abbott Laboratories, and Beckman Coulter, which lead the market with advanced testing solutions and innovative diagnostic technologies.
What are the primary factors driving the growth in the D Dimer industry?
Growth drivers in the D-dimer industry include the increasing prevalence of thromboembolic disorders, the rising demand for rapid diagnostic tests, advancements in technology, and enhanced awareness regarding early diagnosis and management of diseases.
Which region is the fastest Growing in the D Dimer market?
The Asia Pacific region is showing significant growth potential in the D-dimer market, projected to grow from $82.40 million in 2023 to $143.90 million by 2033, reflecting an increasing healthcare infrastructure and demand for diagnostic services.
Does ConsaInsights provide customized market report data for the D Dimer industry?
Yes, ConsaInsights offers customized market report data tailored to client specifications, ensuring comprehensive insights and analysis that meet the precise requirements of businesses in the D-dimer industry.
What deliverables can I expect from this D Dimer market research project?
From the D-dimer market research project, expect deliverables such as detailed market analysis reports, segmentation data, growth forecasts, regional insights, competitive landscape evaluations, and strategic recommendations.
What are the market trends of D Dimer?
Current trends in the D-dimer market include the growing preference for point-of-care testing, increased investments in research and development, rising awareness about thrombosis, and the development of novel rapid testing technologies.