Dendritic Cell Cancer Vaccines Market Report
Published Date: 31 January 2026 | Report Code: dendritic-cell-cancer-vaccines
Dendritic Cell Cancer Vaccines Market Size, Share, Industry Trends and Forecast to 2033
This report investigates the global Dendritic Cell Cancer Vaccines market, offering insights into market trends, size, led by a detailed analysis of various industry segments, regional performance, and key market players. The forecast period ranges from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
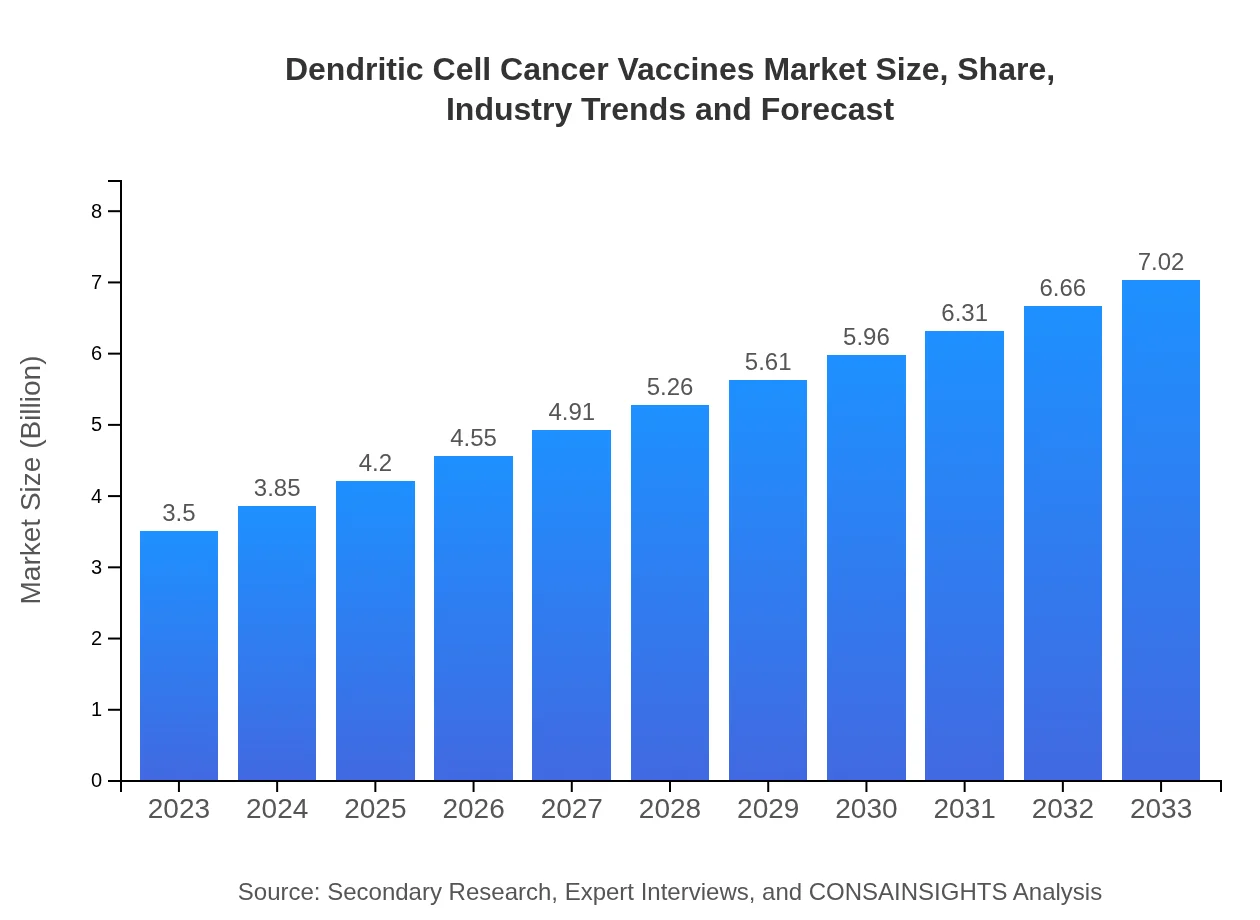
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 7.0% |
| 2033 Market Size | $7.02 Billion |
| Top Companies | Celldex Therapeutics, Inc., BioNTech SE, Dendreon Pharmaceuticals LLC, MediGene AG, GSK plc |
| Last Modified Date | 31 January 2026 |
Dendritic Cell Cancer Vaccines Market Overview
Customize Dendritic Cell Cancer Vaccines Market Report market research report
- ✔ Get in-depth analysis of Dendritic Cell Cancer Vaccines market size, growth, and forecasts.
- ✔ Understand Dendritic Cell Cancer Vaccines's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Dendritic Cell Cancer Vaccines
What is the Market Size & CAGR of Dendritic Cell Cancer Vaccines market in 2023?
Dendritic Cell Cancer Vaccines Industry Analysis
Dendritic Cell Cancer Vaccines Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Dendritic Cell Cancer Vaccines Market Analysis Report by Region
Europe Dendritic Cell Cancer Vaccines Market Report:
Europe is projected to enhance its Dendritic Cell Cancer Vaccines market from $1.01 billion in 2023 to $2.01 billion by 2033. Pioneering research institutions and favorable government regulations regarding cancer treatments are significant contributory factors driving this market expansion across the UK, Germany, and France.Asia Pacific Dendritic Cell Cancer Vaccines Market Report:
In the Asia Pacific region, the Dendritic Cell Cancer Vaccines market is expected to grow from $0.70 billion in 2023 to $1.41 billion by 2033, fueled by increased investment in healthcare infrastructure and growing awareness of personalized medicine. Countries like China and Japan lead in market potential, with rising cancer incidence rates driving demand for innovative therapeutic solutions.North America Dendritic Cell Cancer Vaccines Market Report:
The North American Dendritic Cell Cancer Vaccines market is anticipated to escalate from $1.13 billion in 2023 to $2.27 billion in 2033. Strong infrastructure for research and development, supported by well-established pharmaceutical companies and extensive clinical trials, will boost market growth, reflecting a robust cancer treatment landscape.South America Dendritic Cell Cancer Vaccines Market Report:
The South American market for Dendritic Cell Cancer Vaccines is forecasted to experience growth from $0.20 billion in 2023 to $0.40 billion by 2033. The emphasis on cancer research and growing funding from governmental and non-governmental organizations is expected to fuel market expansion, alongside increased access to advanced therapies in Brazil and Argentina.Middle East & Africa Dendritic Cell Cancer Vaccines Market Report:
The Dendritic Cell Cancer Vaccines market in the Middle East and Africa is estimated to rise from $0.46 billion in 2023 to $0.92 billion by 2033. The region is gradually adopting vaccines backed by collaborative efforts with global healthcare organizations aiming to improve cancer treatment options and care standards in nations like South Africa and UAE.Tell us your focus area and get a customized research report.
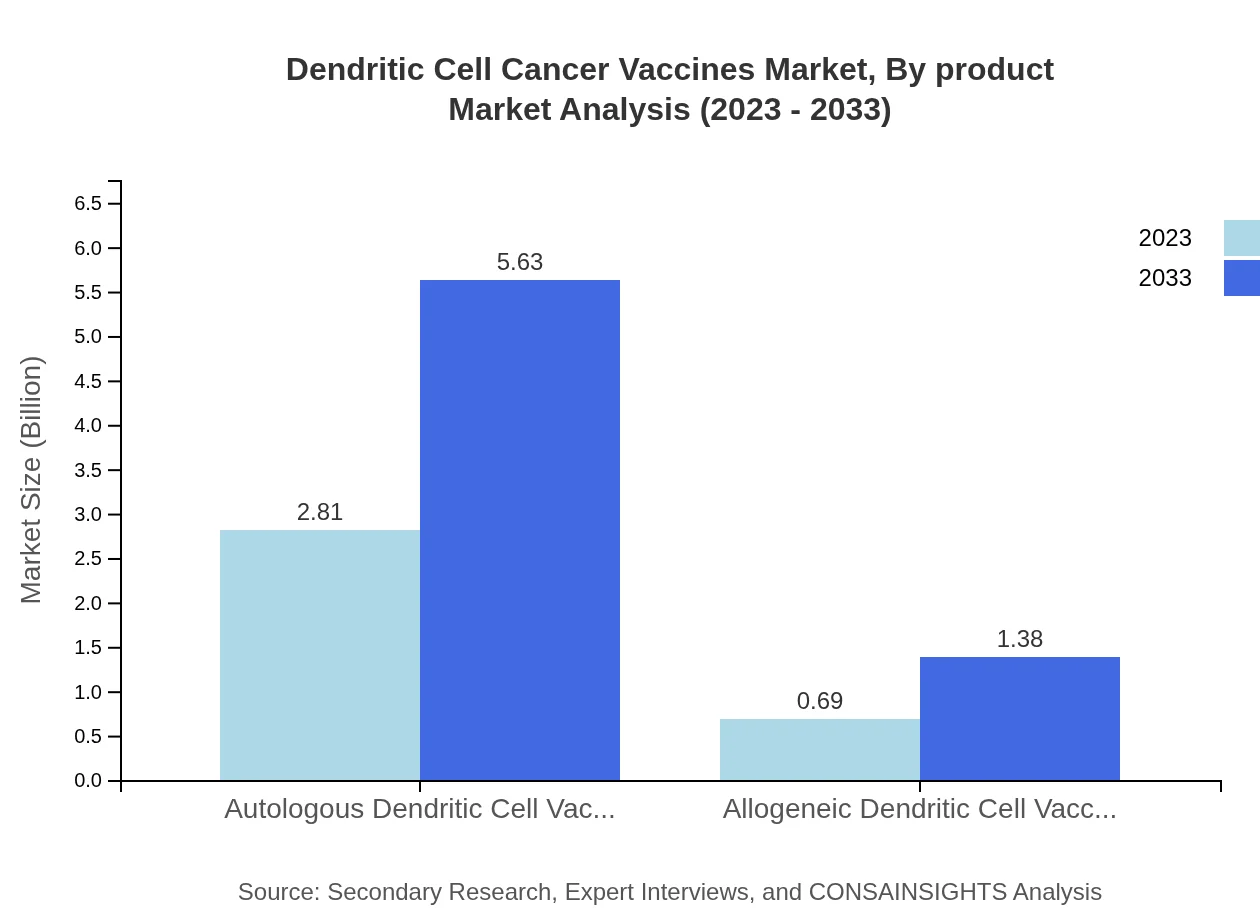
Dendritic Cell Cancer Vaccines Market Analysis By Product
The Dendritic Cell Cancer Vaccines market is dominantly led by Autologous Dendritic Cell Vaccines, with the market size projected to grow from $2.81 billion in 2023 to $5.63 billion by 2033, maintaining a significant market share of 80.32%. In contrast, Allogeneic Dendritic Cell Vaccines are also on the rise, with growth from $0.69 billion to $1.38 billion and holding a market share of 19.68%.
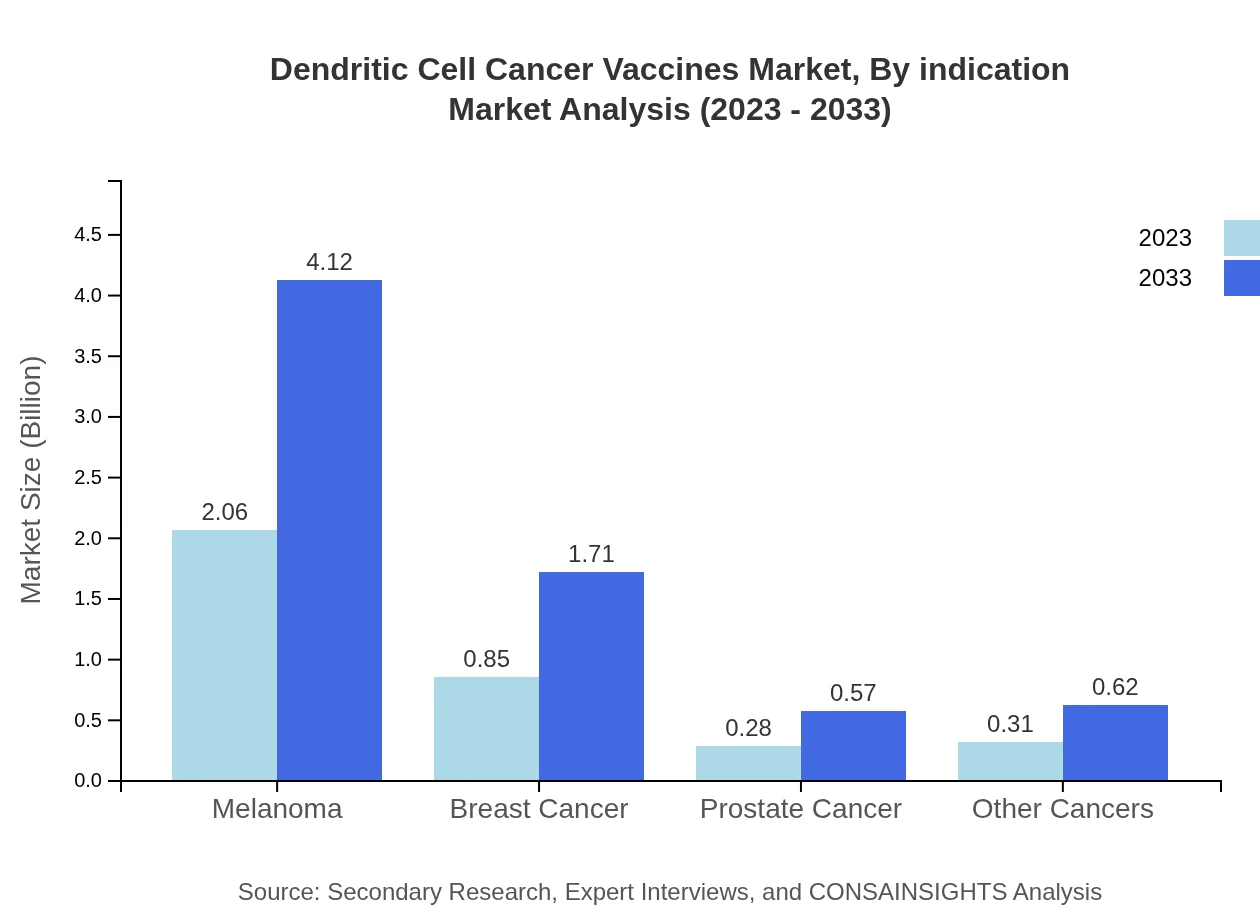
Dendritic Cell Cancer Vaccines Market Analysis By Indication
By indication, the Melanoma segment leads the market with a size of $2.06 billion in 2023, expected to double to $4.12 billion in 2033, capturing a market share of 58.75%. Other significant indications include Breast Cancer and Prostate Cancer, with projected increases from $0.85 billion to $1.71 billion and $0.28 billion to $0.57 billion, respectively.
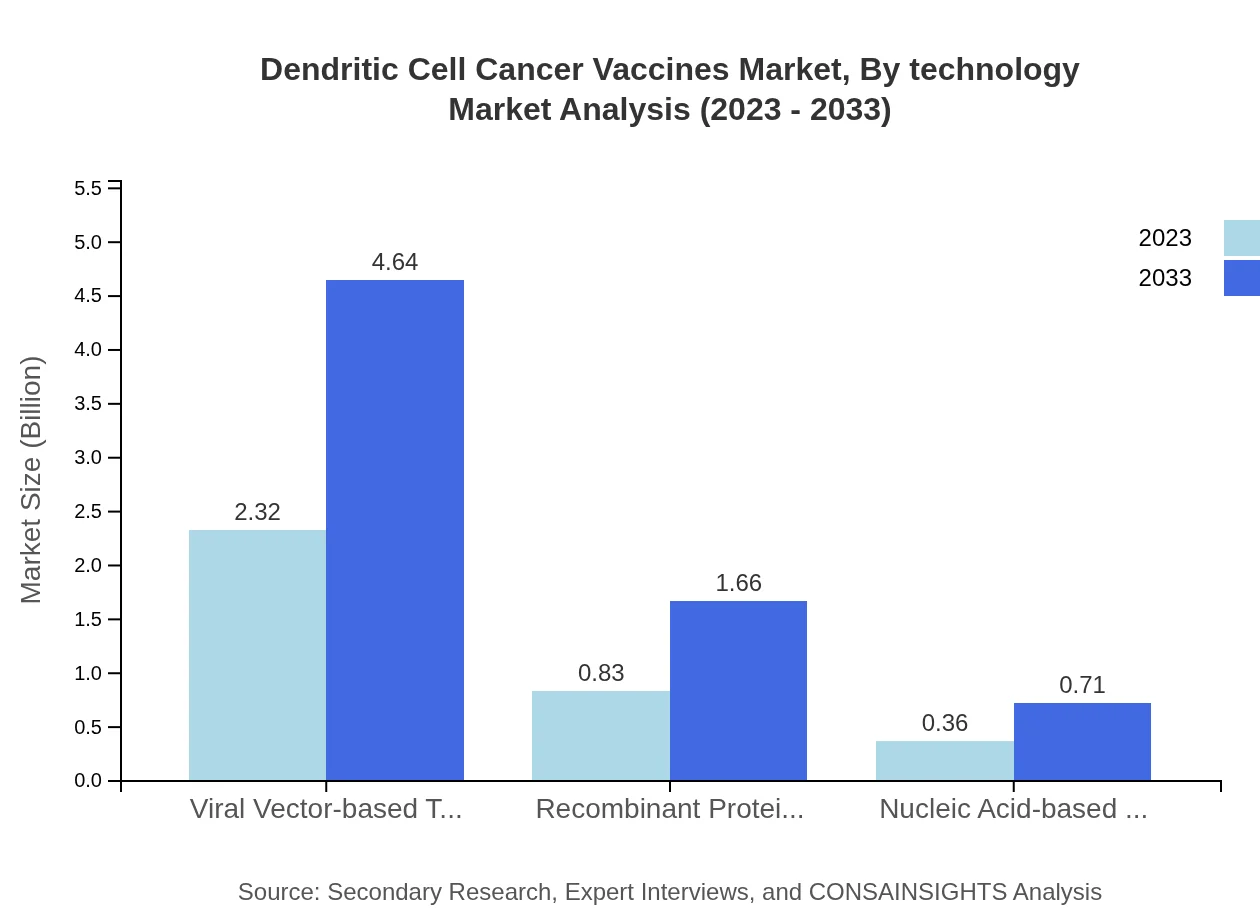
Dendritic Cell Cancer Vaccines Market Analysis By Technology
The technology analysis reveals that Viral Vector-based Technologies dominate the market with a size of $2.32 billion in 2023, expected to grow to $4.64 billion by 2033, holding a market share of 66.16%. Nucleic Acid-based technologies show potential, albeit smaller, growing from $0.36 billion to $0.71 billion, capturing a 10.15% share.
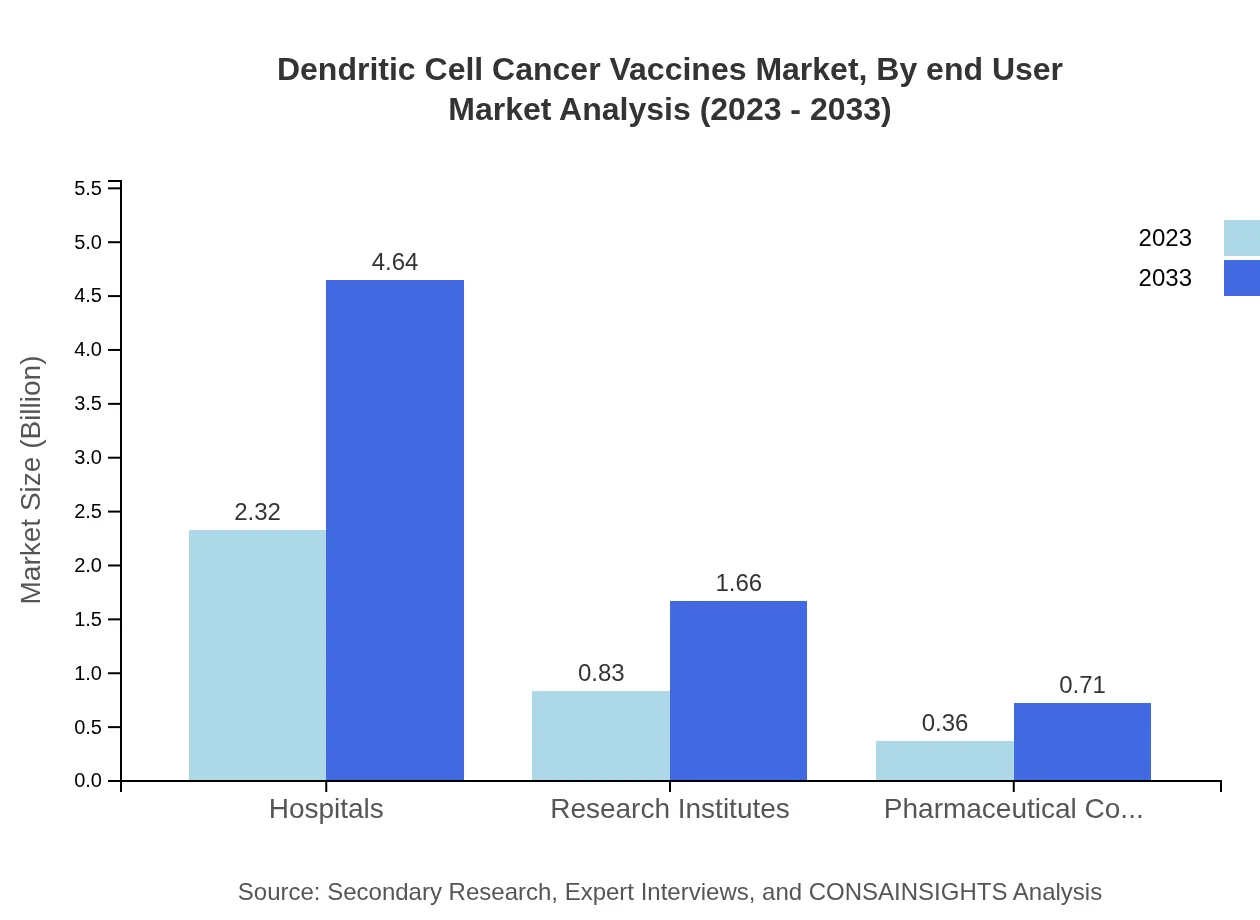
Dendritic Cell Cancer Vaccines Market Analysis By End User
The market by end-user comprises Hospitals, which play a pivotal role, expanding from $2.32 billion in 2023 to $4.64 billion by 2033, holding a 66.16% market share. Research Institutes and Pharmaceutical Companies also contribute significantly, with sizes of $0.83 billion and $0.36 billion in 2023 and expected growth through 2033.
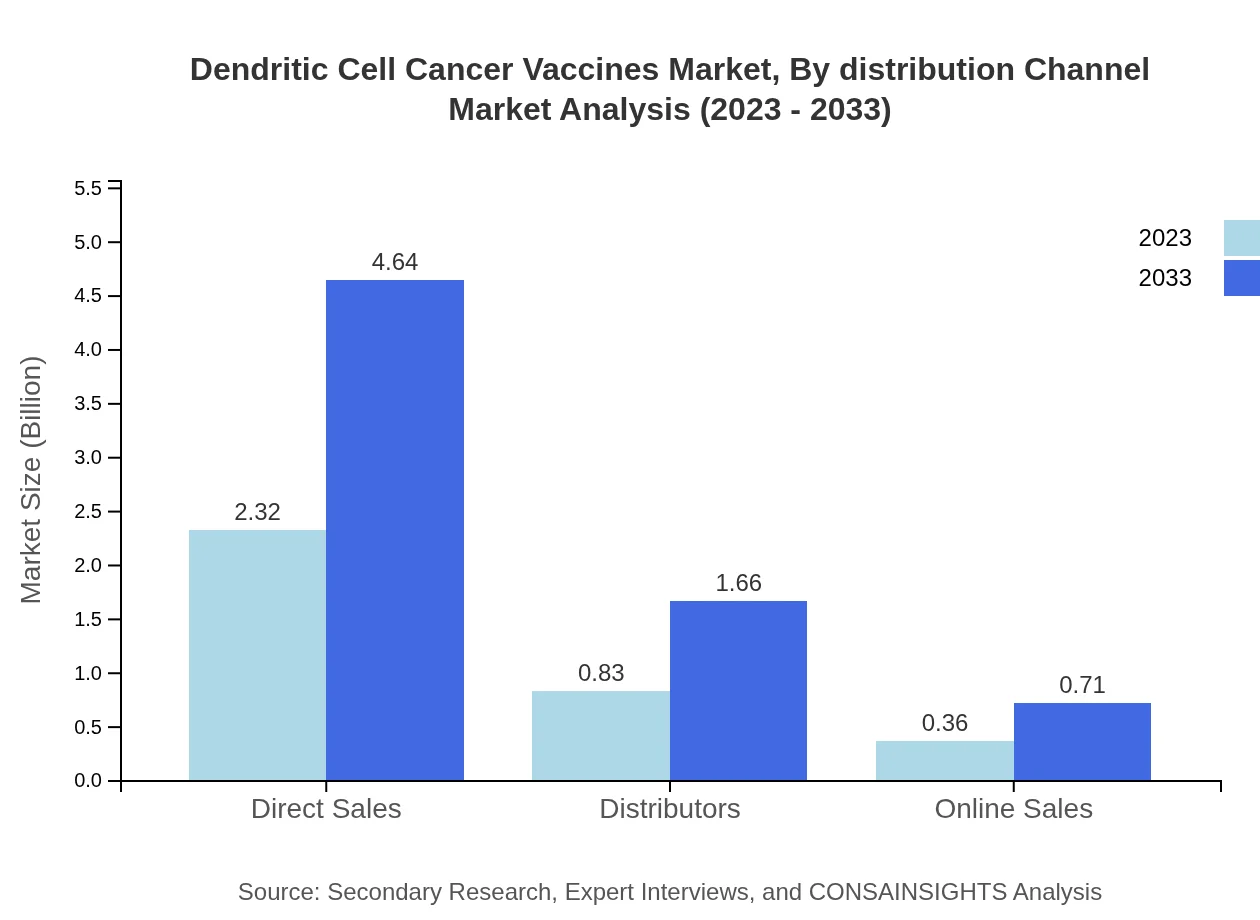
Dendritic Cell Cancer Vaccines Market Analysis By Distribution Channel
The Dendritic Cell Cancer Vaccines market segmented by distribution channel indicates that Direct Sales lead the way from $2.32 billion in 2023 to $4.64 billion in 2033, complemented by Online Sales and Distributors, growing moderately, reflecting current purchasing trends in healthcare.
Dendritic Cell Cancer Vaccines Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Dendritic Cell Cancer Vaccines Industry
Celldex Therapeutics, Inc.:
Celldex Therapeutics is focused on developing targeted therapeutics for cancer treatment, specializing in dendritic cell vaccines that show promise in treating various malignancies.BioNTech SE:
BioNTech uses innovative messenger RNA technology to develop targeted cancer therapies, including dendritic cell vaccines, that have progressed into advanced clinical trials.Dendreon Pharmaceuticals LLC:
Pioneers in the industry, Dendreon developed the first FDA-approved dendritic cell vaccine for prostate cancer, demonstrating significant patient benefits during clinical studies.MediGene AG:
MediGene focuses on developing and advancing individualized cancer immunotherapies, including dendritic cell vaccines aimed at enhancing therapeutic outcomes.GSK plc:
GSK is a global professional in pharmaceutical and healthcare products, working on innovative solutions, including dendritic cell vaccines for cancer treatment.We're grateful to work with incredible clients.









FAQs
What is the market size of dendritic Cell Cancer Vaccines?
The dendritic cell cancer vaccines market is currently valued at approximately $3.5 billion, with a projected CAGR of 7.0% from 2023 to 2033, indicating significant potential for growth and investment.
What are the key market players or companies in this dendritic Cell Cancer Vaccines industry?
The dendritic cell cancer vaccines market includes key players such as Dendreon Corporation, Northwest Biotherapeutics, and Celldex Therapeutics, which are leading advancements and innovations in vaccine development.
What are the primary factors driving the growth in the dendritic Cell Cancer Vaccines industry?
Factors driving market growth include increasing cancer prevalence, advancements in cancer immunotherapy, growing awareness of personalized medicine, and strong government funding for innovative treatment research.
Which region is the fastest Growing in the dendritic Cell Cancer Vaccines?
North America is the fastest-growing region in the dendritic cell cancer vaccines market, expected to grow from $1.13 billion in 2023 to $2.27 billion by 2033, showcasing robust research and development efforts.
Does ConsaInsights provide customized market report data for the dendritic Cell Cancer Vaccines industry?
Yes, ConsaInsights offers customized market report data for the dendritic cell cancer vaccines industry, tailoring insights and analyses according to client specifications and requirements.
What deliverables can I expect from this dendritic Cell Cancer Vaccines market research project?
Deliverables include an in-depth report on market trends, detailed segmentation analysis, competitive landscape assessments, and forecasts, along with strategic recommendations and actionable insights.
What are the market trends of dendritic Cell Cancer Vaccines?
Key trends in the dendritic cell cancer vaccines market include the rising trend of personalized cancer therapies, increasing investment in R&D, and a shift towards combination therapies that integrate vaccines with other treatment modalities.