Dengue Virus Diagnostic Tests Market Report
Published Date: 31 January 2026 | Report Code: dengue-virus-diagnostic-tests
Dengue Virus Diagnostic Tests Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Dengue Virus Diagnostic Tests market, covering an overview, size, growth rate, regional insights, and key trends from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
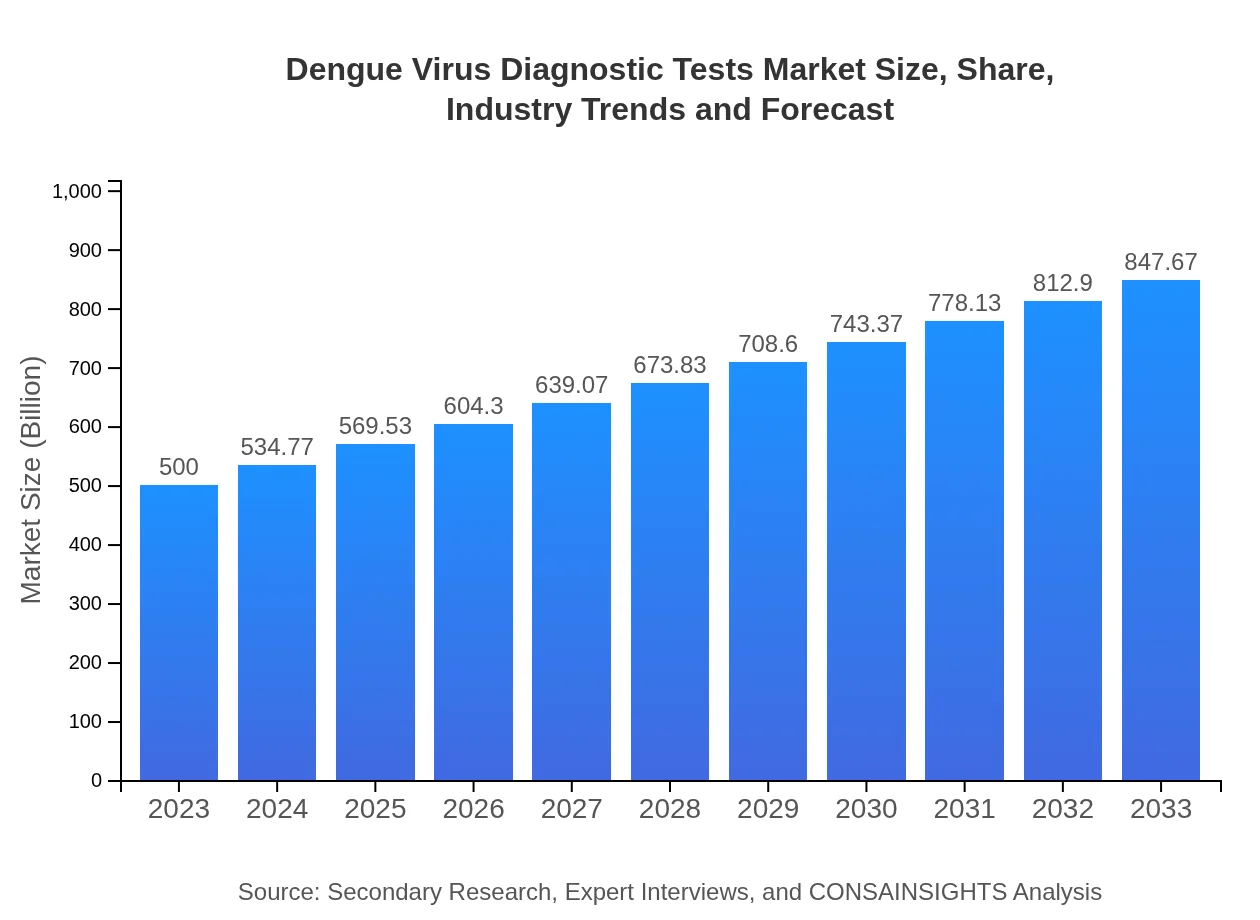
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.3% |
| 2033 Market Size | $847.67 Million |
| Top Companies | Roche Diagnostics, Abbott Laboratories, SD Biosensor, InBios International, Inc. |
| Last Modified Date | 31 January 2026 |
Dengue Virus Diagnostic Tests Market Overview
Customize Dengue Virus Diagnostic Tests Market Report market research report
- ✔ Get in-depth analysis of Dengue Virus Diagnostic Tests market size, growth, and forecasts.
- ✔ Understand Dengue Virus Diagnostic Tests's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Dengue Virus Diagnostic Tests
What is the Market Size & CAGR of Dengue Virus Diagnostic Tests market in 2023?
Dengue Virus Diagnostic Tests Industry Analysis
Dengue Virus Diagnostic Tests Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Dengue Virus Diagnostic Tests Market Analysis Report by Region
Europe Dengue Virus Diagnostic Tests Market Report:
The European market is projected to rise from USD 146.45 million in 2023 to USD 248.28 million by 2033. Awareness campaigns and collaborations among public health organizations are pivotal in fostering demand for effective diagnostic tests in Europe.Asia Pacific Dengue Virus Diagnostic Tests Market Report:
The Asia Pacific region is expected to see substantial growth in the Dengue Virus Diagnostic Tests market, with a market size projected to increase from USD 89.90 million in 2023 to USD 152.41 million by 2033. This growth is driven by high dengue prevalence in countries like India, Indonesia, and the Philippines, necessitating effective diagnostic solutions.North America Dengue Virus Diagnostic Tests Market Report:
North America is anticipated to grow from USD 194.35 million in 2023 to USD 329.49 million by 2033. The region benefits from advanced healthcare systems, high laboratory testing rates, and increasing preventive measures against vector-borne diseases.South America Dengue Virus Diagnostic Tests Market Report:
In South America, the market is forecasted to grow from USD 34.55 million in 2023 to USD 58.57 million in 2033. Factors driving this market include rising awareness of dengue health risks and increasing investments in healthcare infrastructure in countries like Brazil and Colombia.Middle East & Africa Dengue Virus Diagnostic Tests Market Report:
The market in the Middle East and Africa is expected to see growth from USD 34.75 million in 2023 to USD 58.91 million by 2033. Challenges related to healthcare infrastructure are being addressed through international health initiatives which enhance the capabilities for dengue testing.Tell us your focus area and get a customized research report.
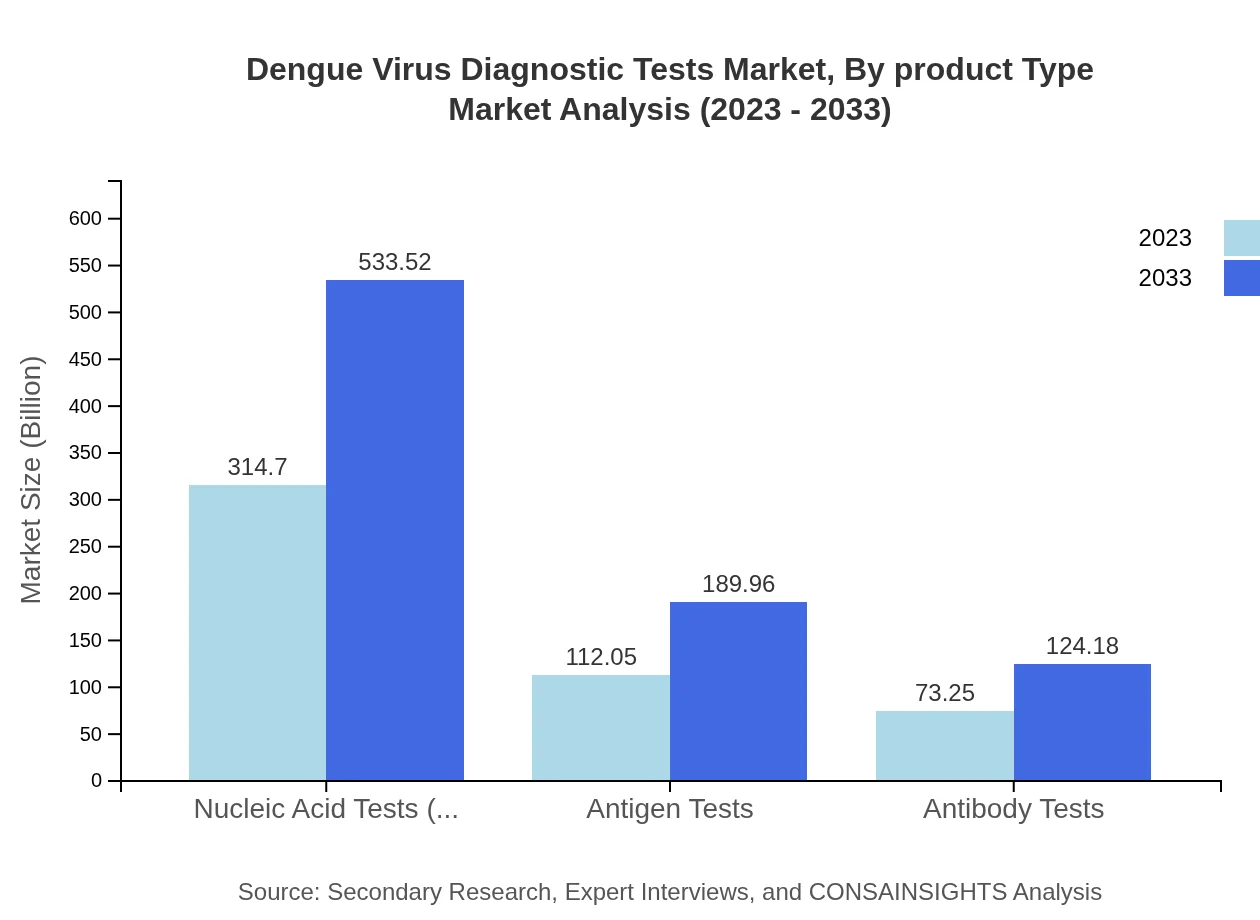
Dengue Virus Diagnostic Tests Market Analysis By Product Type
In terms of product type, the Molecular Diagnostics segment will dominate with a projected market size increase from USD 314.70 million in 2023 to USD 533.52 million by 2033, representing a significant share of the overall market. Serological tests and rapid tests will follow, with respective market sizes growing from USD 112.05 million to USD 189.96 million and USD 73.25 million to USD 124.18 million.
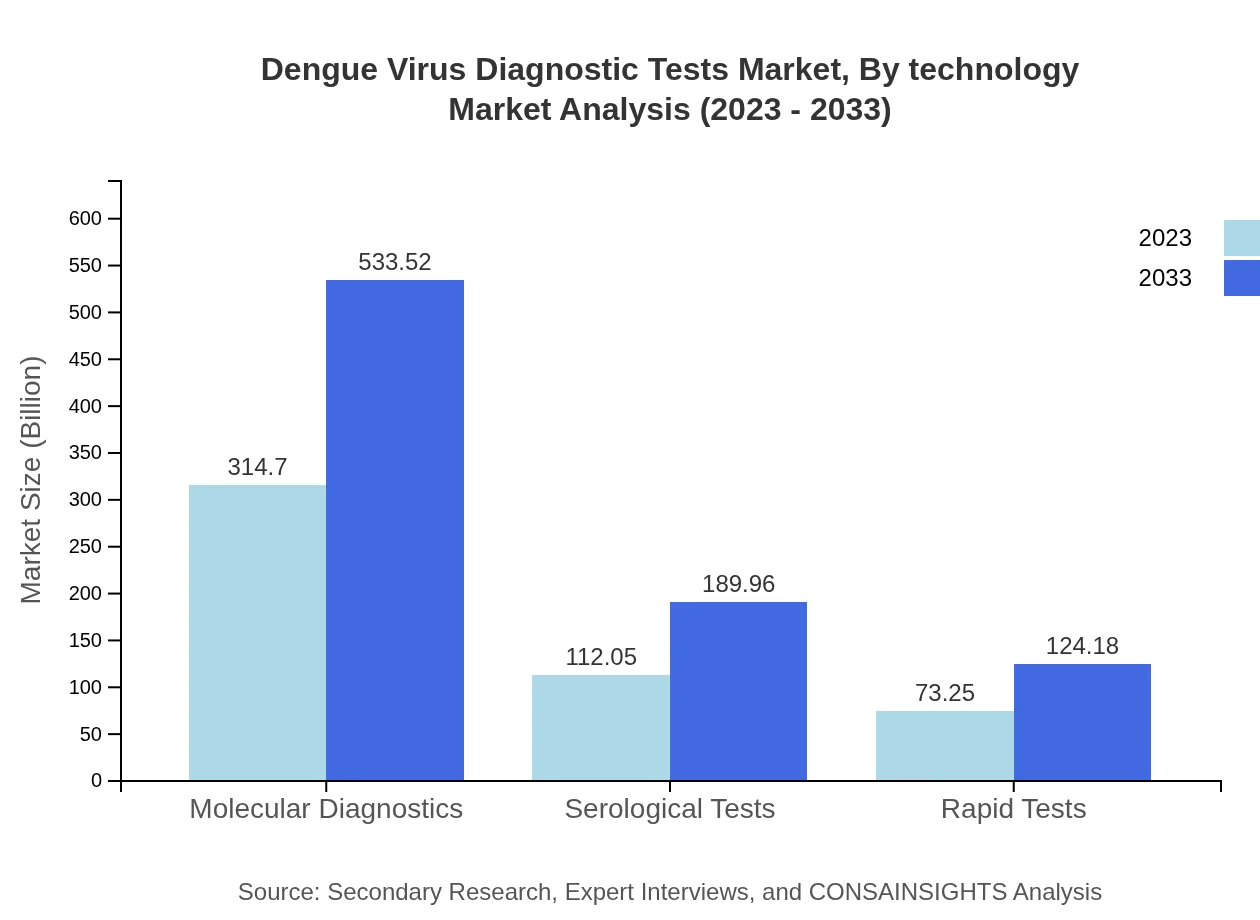
Dengue Virus Diagnostic Tests Market Analysis By Technology
The market segments by technology are characterized by molecular diagnostics, serological testing, and rapid test kits. Molecular diagnostics, being the most advanced method, offer superior sensitivity and specificity, expected to capture more than 60% market share throughout the forecast period.
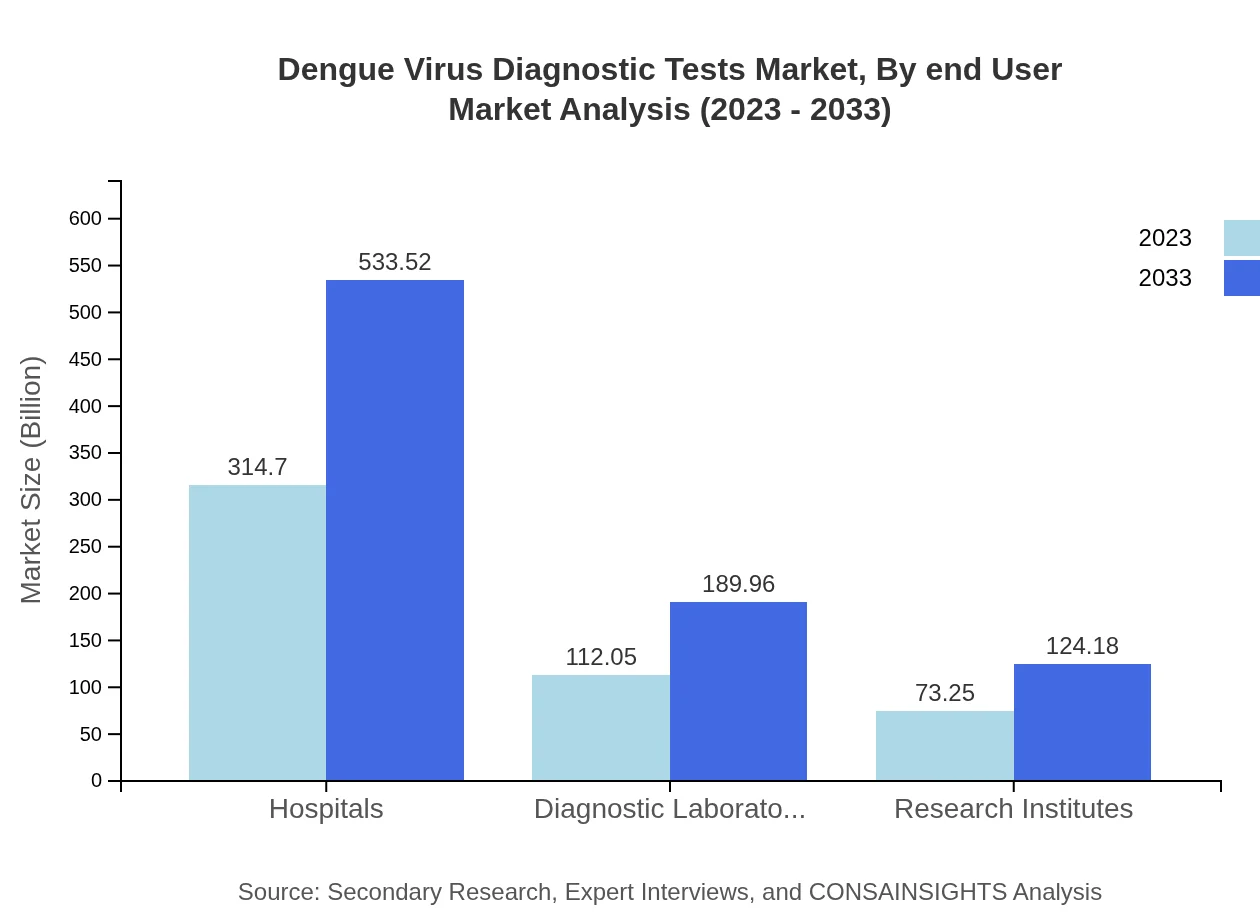
Dengue Virus Diagnostic Tests Market Analysis By End User
Hospitals will emerge as the leading end-user segment, expanding from a market size of USD 314.70 million in 2023 to USD 533.52 million by 2033, maintaining a robust share in the market. Diagnostic laboratories and research institutes will also significantly contribute to the market growth.
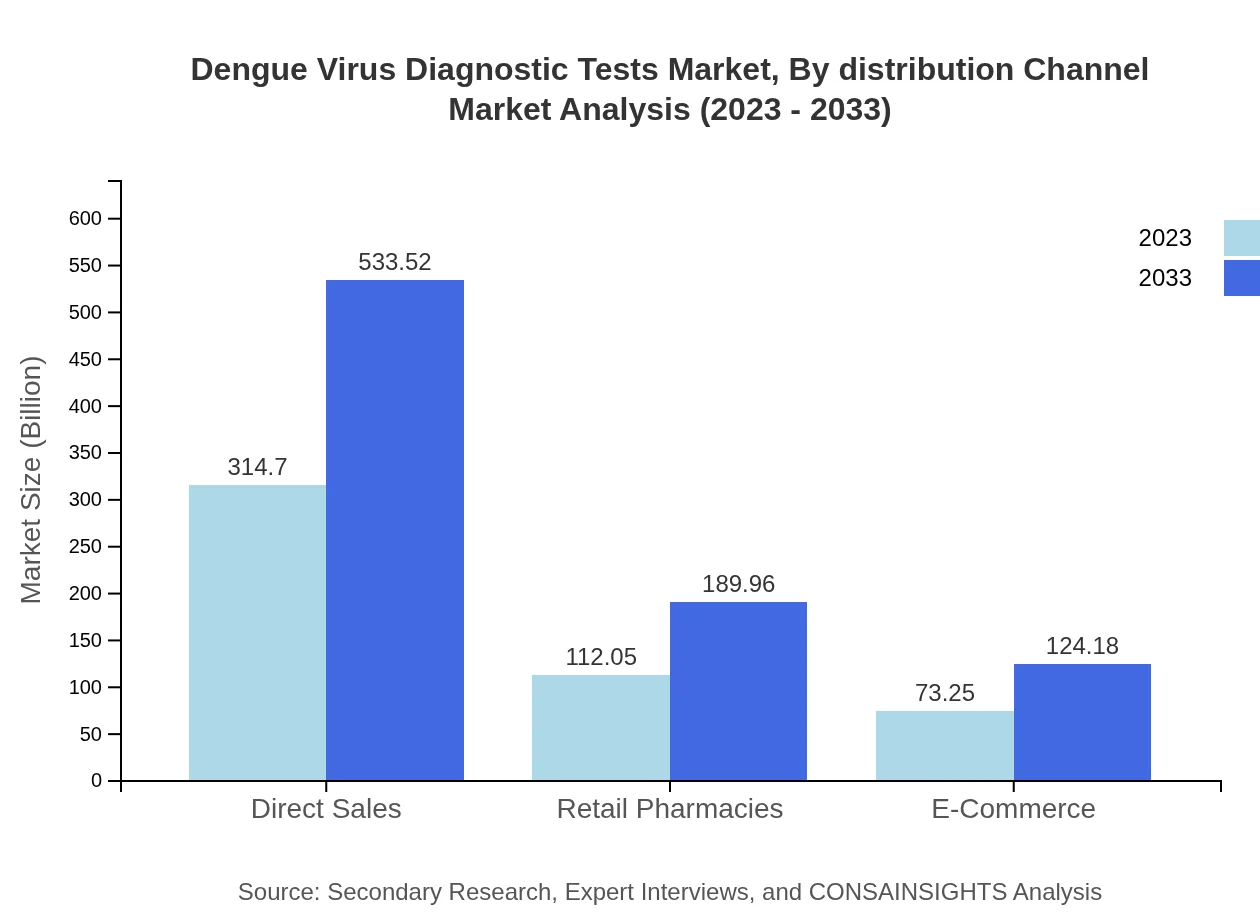
Dengue Virus Diagnostic Tests Market Analysis By Distribution Channel
Direct sales are projected to retain leading market share in distribution, while retail pharmacies and e-commerce are also witnessing growth, reflecting changing consumer purchasing patterns toward convenience and accessibility of diagnostic tests.
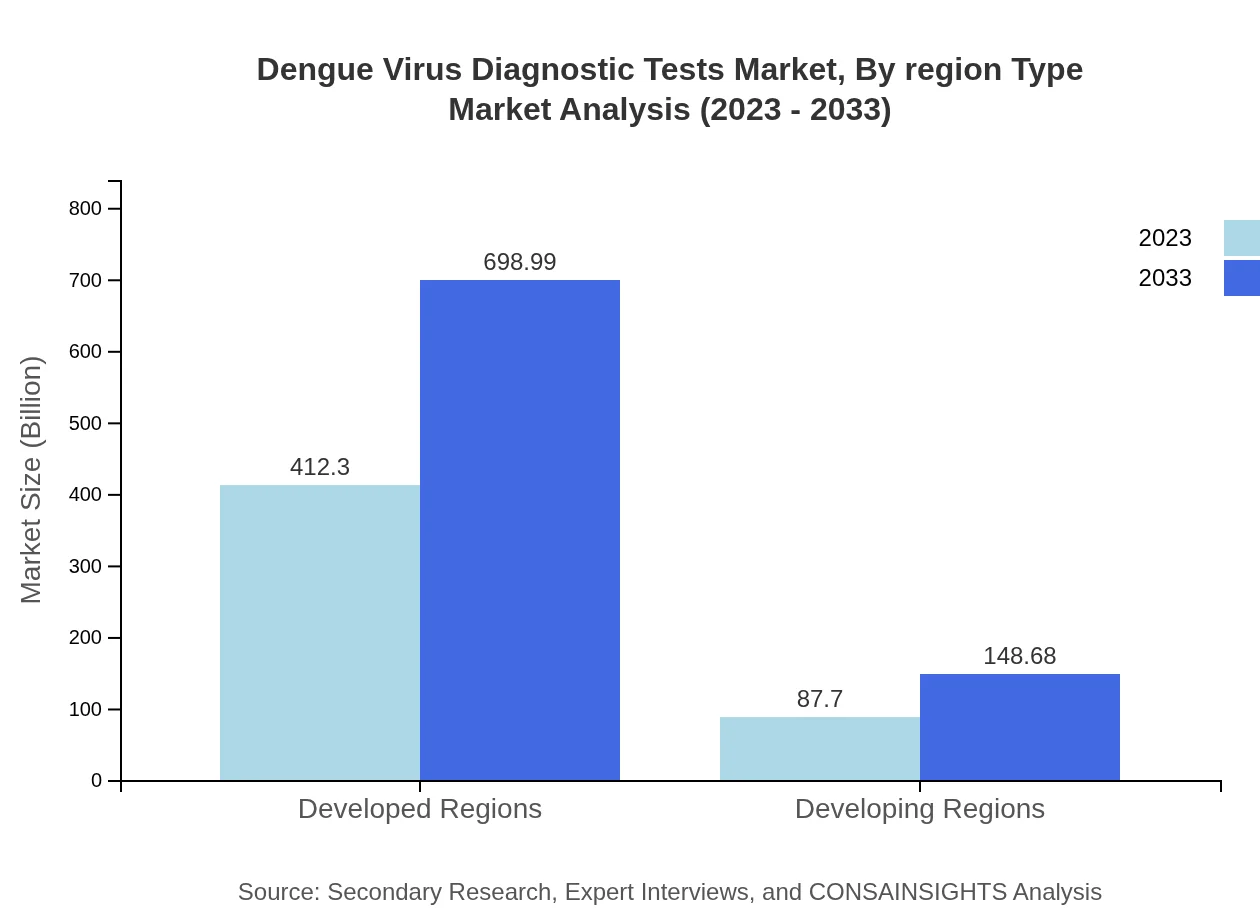
Dengue Virus Diagnostic Tests Market Analysis By Region Type
The market analysis by region indicates developed regions outpacing developing regions in market size, expected to grow from USD 412.30 million to USD 698.99 million from 2023 to 2033, demonstrating the concentration of healthcare resources and demand for high-quality diagnostic tests.
Dengue Virus Diagnostic Tests Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Dengue Virus Diagnostic Tests Industry
Roche Diagnostics:
A leading player providing comprehensive diagnostics solutions and has a robust dengue diagnostic portfolio, focusing on high sensitivity and specificity.Abbott Laboratories:
Known for its innovation in diagnostic testing, Abbott offers rapid and molecular testing solutions for dengue diagnostics, contributing to improved patient outcomes.SD Biosensor:
Specializes in developing rapid diagnostic tests and has made significant contributions to the availability of dengue tests with quick turnaround times.InBios International, Inc.:
A biotechnology company focused on creating serological and ELISA tests for dengue, emphasizing ease of use and accuracy.We're grateful to work with incredible clients.









FAQs
What is the market size of dengue Virus Diagnostic Tests?
The dengue virus diagnostic tests market is projected to reach approximately $500 million by 2033, growing at a CAGR of 5.3%. This growth reflects increasing cases of dengue and the need for effective diagnostic solutions.
What are the key market players or companies in this dengue Virus Diagnostic Tests industry?
Key players in the dengue virus diagnostic tests market include established companies such as Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers. These firms are recognized for their innovative diagnostic technologies and broad distribution networks.
What are the primary factors driving the growth in the dengue Virus Diagnostic Tests industry?
Growth in the dengue diagnostic tests industry is driven by rising incidence rates of dengue fever, technological advancements in diagnostic methods, and increasing investments in healthcare infrastructure, particularly in endemic regions.
Which region is the fastest Growing in the dengue Virus Diagnostic Tests?
The Asia-Pacific region is the fastest-growing market for dengue virus diagnostic tests, expected to expand from $89.90 million in 2023 to $152.41 million by 2033. This growth is due to higher prevalence rates and enhanced healthcare access.
Does ConsaInsights provide customized market report data for the dengue Virus Diagnostic Tests industry?
Yes, ConsaInsights offers customized market report data tailored to client needs in the dengue virus diagnostic tests industry. Clients can request specific insights, statistics, and forecasts relevant to their business objectives.
What deliverables can I expect from this dengue Virus Diagnostic Tests market research project?
Deliverables from the dengue virus diagnostic tests market research include comprehensive market analysis, trend insights, competitive landscape evaluations, and precise forecasts segmented by region and product type.
What are the market trends of dengue Virus Diagnostic Tests?
Market trends in dengue virus diagnostic tests include increasing adoption of molecular testing, growth in rapid test utilization for field deployment, and a shift towards digital diagnostics platforms to enhance efficiency in disease management.