Diabetic Macular Edema Market Report
Published Date: 31 January 2026 | Report Code: diabetic-macular-edema
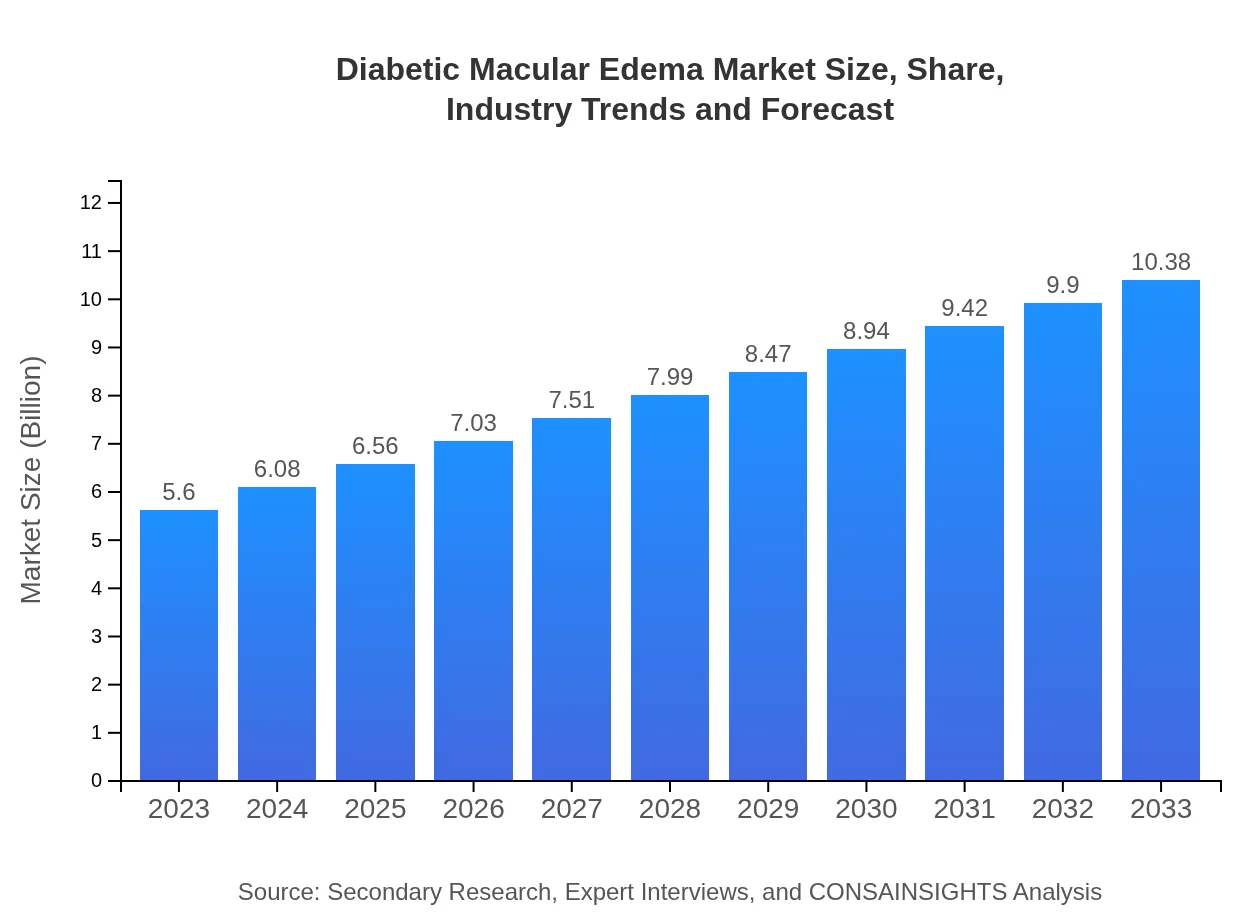
Diabetic Macular Edema Market Size, Share, Industry Trends and Forecast to 2033
This report analyzes the Diabetic Macular Edema market from 2023 to 2033, providing insights into market size, growth potential, segmentation, regional analyses, and industry dynamics. It aims to offer actionable data for stakeholders in this rapidly evolving sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $10.38 Billion |
| Top Companies | Regeneron Pharmaceuticals, Novartis AG, Roche |
| Last Modified Date | 31 January 2026 |
Diabetic Macular Edema Market Overview
Customize Diabetic Macular Edema Market Report market research report
- ✔ Get in-depth analysis of Diabetic Macular Edema market size, growth, and forecasts.
- ✔ Understand Diabetic Macular Edema's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Diabetic Macular Edema
What is the Market Size & CAGR of Diabetic Macular Edema market in 2023?
Diabetic Macular Edema Industry Analysis
Diabetic Macular Edema Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Diabetic Macular Edema Market Analysis Report by Region
Europe Diabetic Macular Edema Market Report:
Europe's DME market is expected to grow from $1.54 billion in 2023 to $2.86 billion by 2033. Increased funding for diabetic research and a robust regulatory framework favor competitive drug development, enhancing treatment options for patients.Asia Pacific Diabetic Macular Edema Market Report:
The Asia Pacific DME market is projected to grow from $1.17 billion in 2023 to $2.17 billion by 2033, driven by increasing diabetes prevalence and improved healthcare infrastructure. Japan, China, and India are key markets, with substantial investments in healthcare amenities and awareness programs.North America Diabetic Macular Edema Market Report:
North America accounts for a significant share of the DME market, estimated at $1.97 billion in 2023 and projected to reach $3.65 billion by 2033. The region's advanced healthcare system, high prevalence of diabetes, and rapid adoption of innovative treatment options are key growth drivers.South America Diabetic Macular Edema Market Report:
In South America, the DME market is expected to expand from $0.40 billion in 2023 to $0.75 billion in 2033. Growing public health initiatives aimed at diabetes management alongside increasing accessibility to healthcare facilities will support this growth.Middle East & Africa Diabetic Macular Edema Market Report:
The Middle East and Africa market is anticipated to grow from $0.51 billion in 2023 to $0.94 billion by 2033. Rising awareness around diabetes and its complications, as well as improving healthcare access in the region, will contribute to market growth.Tell us your focus area and get a customized research report.
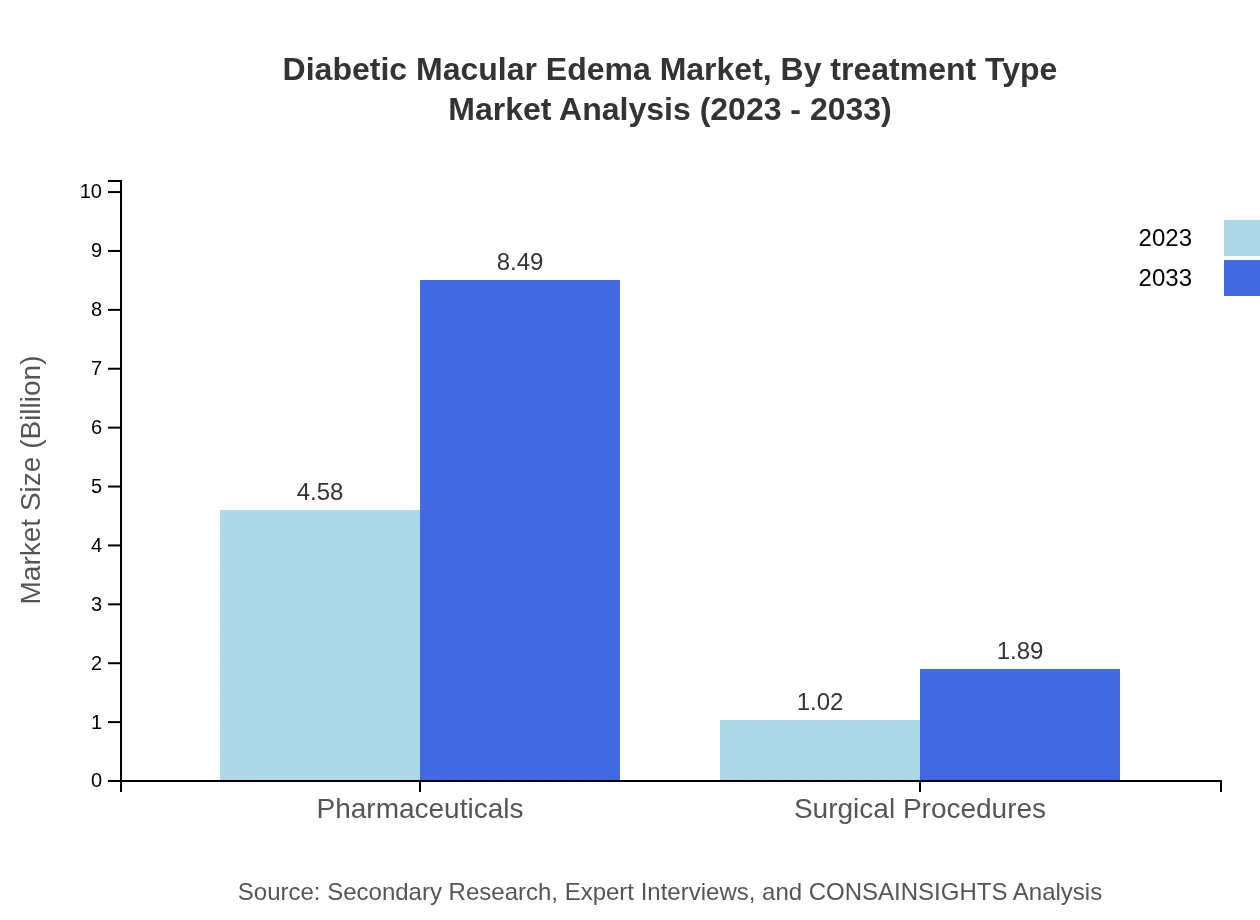
Diabetic Macular Edema Market Analysis By Treatment Type
In 2023, intravitreal injections dominate the treatment segment, valued at $4.58 billion, with a significant market share of 81.81%. This segment is projected to grow to approximately $8.49 billion by 2033. Hospitals account for the largest share of the market due to their ability to provide comprehensive care for DME patients. Surgical procedures, while smaller in market size compared to pharmaceuticals, are crucial for specific patient populations requiring invasive intervention.
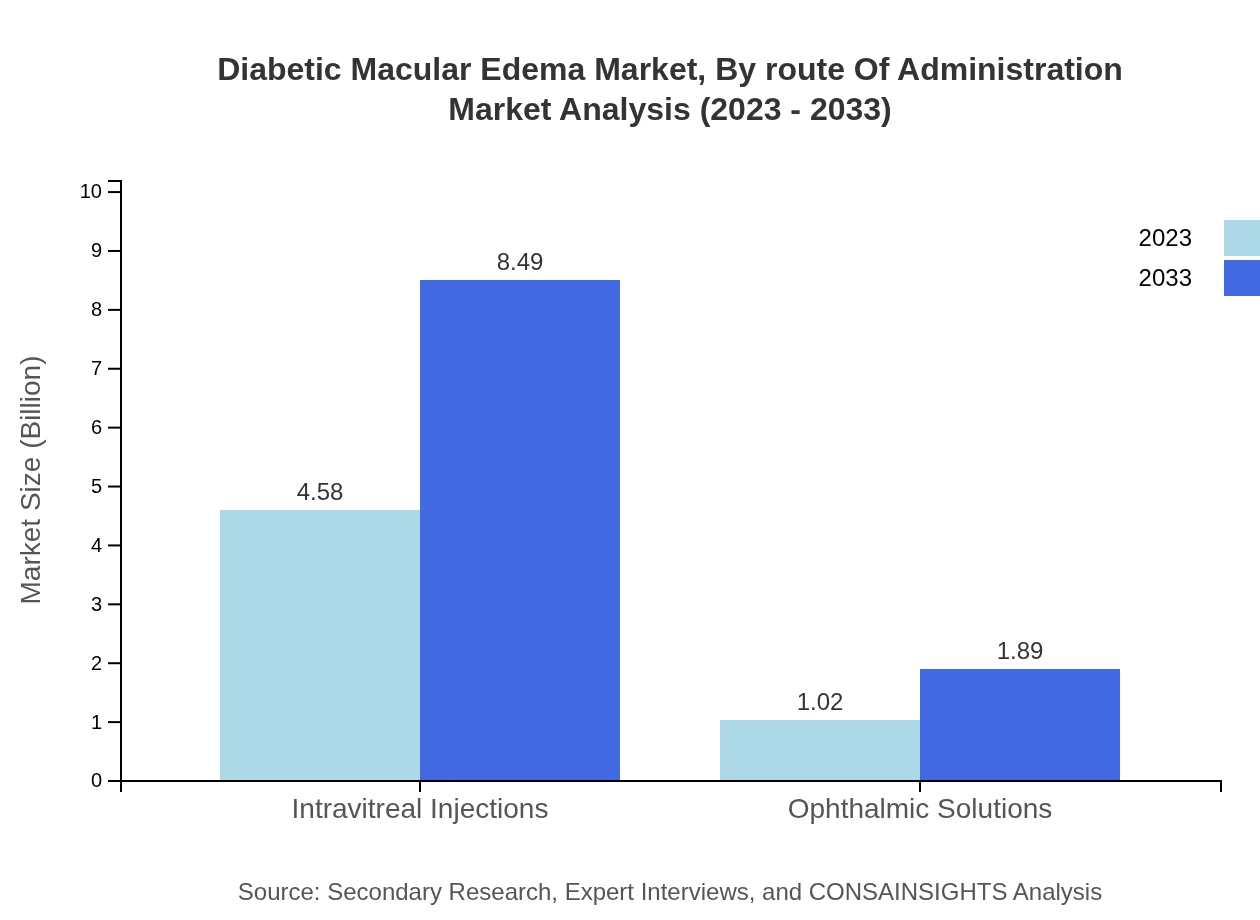
Diabetic Macular Edema Market Analysis By Route Of Administration
Intravitreal administration stands as the primary route for diabetic macular edema therapies, contributing significantly to the market size in this area. The effectiveness and targeted delivery of treatment through this method enhance patient outcomes. Ophthalmic solutions administered topically are also gaining traction, accounting for 18.19% of the market share, emphasizing a growing trend towards less invasive treatment approaches.
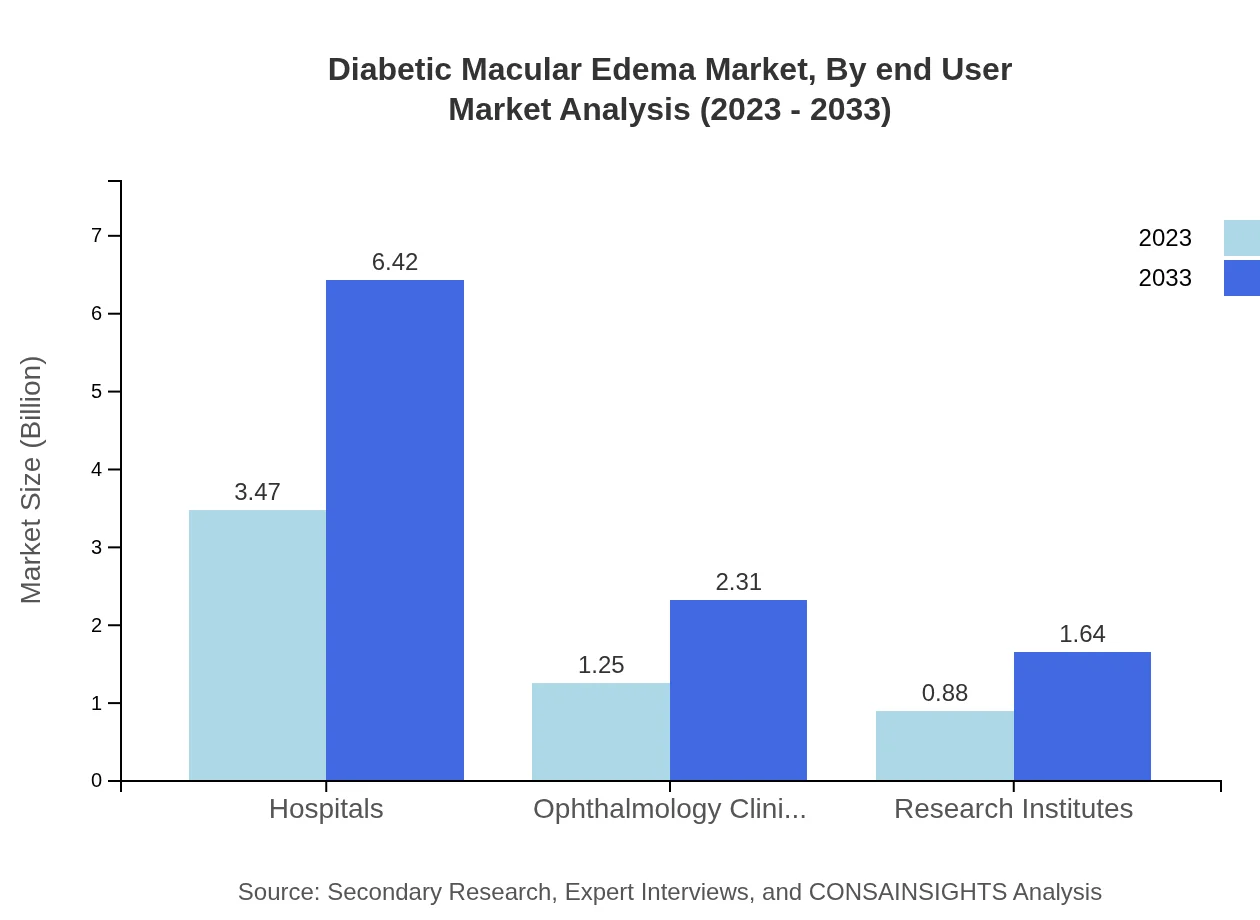
Diabetic Macular Edema Market Analysis By End User
In 2023, hospital settings are leading in the Diabetic Macular Edema treatment space, taking a share of 61.9%. This reflects the demand for specialized care and comprehensive treatment protocols within hospital environments. Ophthalmology clinics and research institutes are also significant players in the market, focusing on outpatient treatments and clinical research endeavors, respectively.
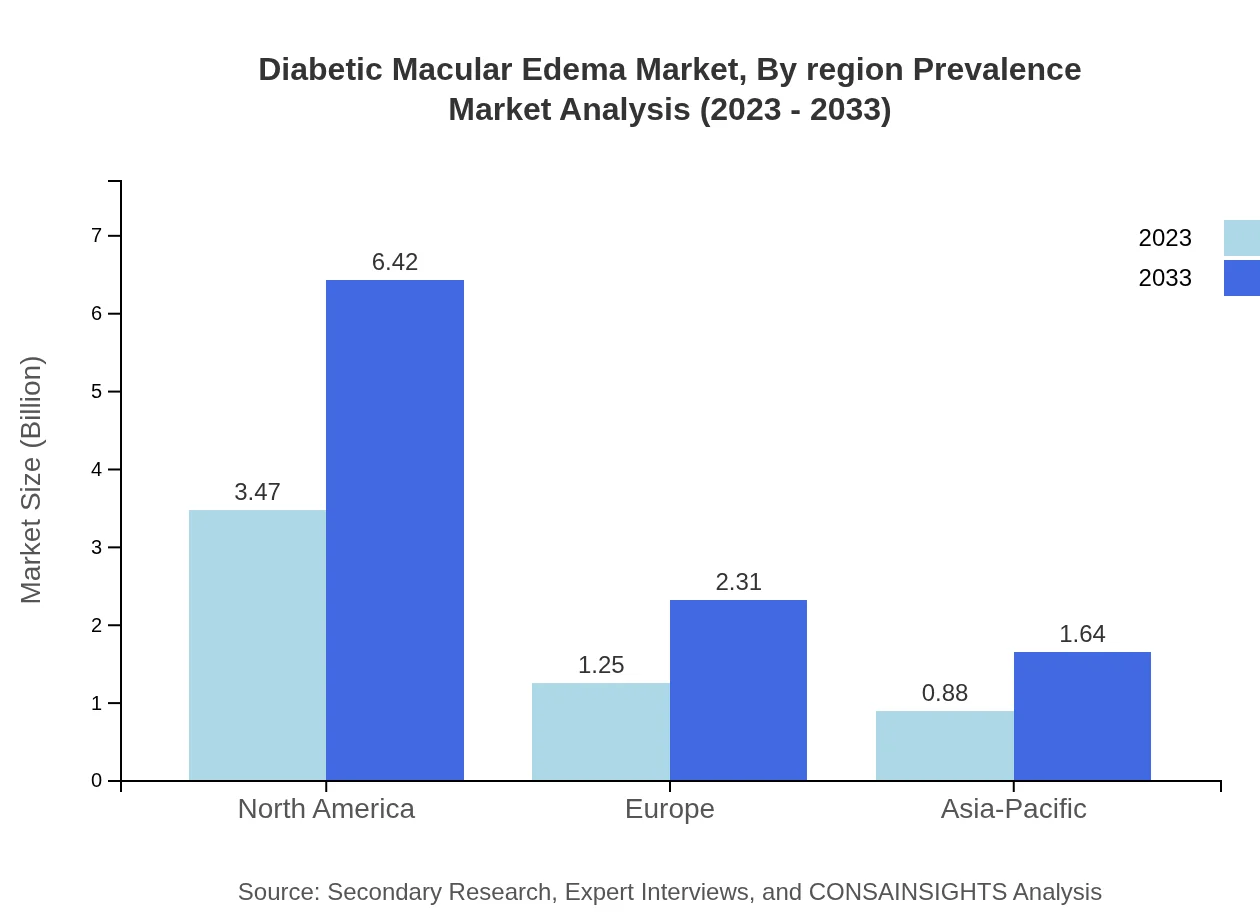
Diabetic Macular Edema Market Analysis By Region Prevalence
North America has the highest prevalence of DME, contributing significantly to the overall market size. Europe follows closely, fueled by extensive diabetic healthcare programs. The Asia Pacific region is witnessing increasing prevalence, anticipated to drive market growth through 2033, indicating a need for targeted healthcare strategies.
Diabetic Macular Edema Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Diabetic Macular Edema Industry
Regeneron Pharmaceuticals:
Regeneron is a leader in developing innovative therapies for treating eye diseases, particularly with their flagship product, Eylea, used extensively in the treatment of diabetic macular edema.Novartis AG:
Novartis has a strong portfolio in ophthalmic medications and is known for its comprehensive approach to managing diabetic eye diseases, contributing significantly to the DME market.Roche:
Roche is involved in biopharmaceuticals and diagnostics, focusing on high-quality proteins and antibody-based therapies for ophthalmological conditions, including diabetic macular edema.We're grateful to work with incredible clients.









FAQs
What is the market size of diabetic Macular Edema?
The diabetic macular edema market is projected to reach USD 5.6 billion by 2033, growing at a CAGR of 6.2%. This growth reflects an increasing prevalence of diabetes and advancements in treatment options.
What are the key market players or companies in this diabetic Macular Edema industry?
Key players in the diabetic macular edema market include major pharmaceutical companies such as Novartis, Roche, Regeneron, and Bayer. These companies lead in the development and distribution of innovative therapies and treatments for diabetic eye diseases.
What are the primary factors driving the growth in the diabetic Macular Edema industry?
Growth in the diabetic macular edema market is driven by factors including rising diabetes prevalence, advancements in medical technologies, increased awareness about eye care, and significant investments in research and development of new therapeutic solutions.
Which region is the fastest Growing in the diabetic Macular Edema?
North America is the fastest-growing region in the diabetic macular edema market, with its size projected to increase from USD 1.97 billion in 2023 to USD 3.65 billion by 2033, driven by high healthcare expenditure and advanced treatment options.
Does ConsaInsights provide customized market report data for the diabetic Macular Edema industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the diabetic macular edema industry, enabling stakeholders to obtain targeted insights based on market dynamics and strategic objectives.
What deliverables can I expect from this diabetic Macular Edema market research project?
Deliverables from the diabetic macular edema market research project include comprehensive reports with market size data, growth forecasts, competitive analysis, and insights on regional trends and therapeutic innovations.
What are the market trends of diabetic Macular Edema?
Current trends in the diabetic macular edema market include a shift towards biologic therapies, increased utilization of intravitreal injections, and a growing focus on personalized medicine to enhance treatment efficacy and patient outcomes.