Diagnostics Enzyme Market Report
Published Date: 31 January 2026 | Report Code: diagnostics-enzyme
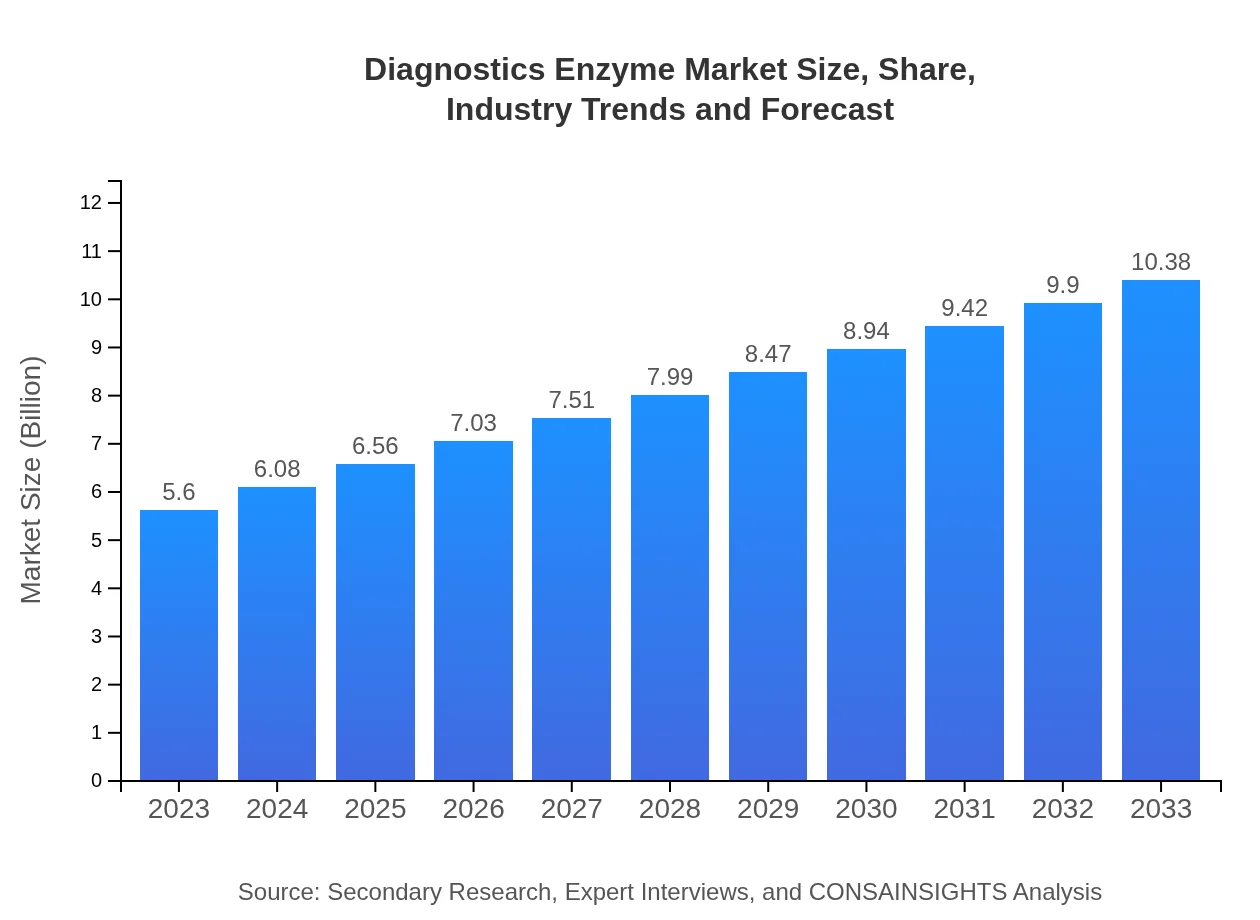
Diagnostics Enzyme Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Diagnostics Enzyme market from 2023 to 2033, covering market size, growth trends, segmentation, regional insights, and key industry players. Detailed forecasts and insights into market dynamics are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $10.38 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Danaher Corporation |
| Last Modified Date | 31 January 2026 |
Diagnostics Enzyme Market Overview
Customize Diagnostics Enzyme Market Report market research report
- ✔ Get in-depth analysis of Diagnostics Enzyme market size, growth, and forecasts.
- ✔ Understand Diagnostics Enzyme's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Diagnostics Enzyme
What is the Market Size & CAGR of Diagnostics Enzyme market in 2023 and 2033?
Diagnostics Enzyme Industry Analysis
Diagnostics Enzyme Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Diagnostics Enzyme Market Analysis Report by Region
Europe Diagnostics Enzyme Market Report:
Europe's Diagnostics Enzyme market is anticipated to rise from $1.47 billion in 2023 to $2.73 billion by 2033. The region benefits from robust healthcare systems, extensive regulatory frameworks, and high demand for innovative diagnostic solutions.Asia Pacific Diagnostics Enzyme Market Report:
In the Asia Pacific region, the Diagnostics Enzyme market is expected to grow from $1.17 billion in 2023 to $2.17 billion by 2033, driven by an increase in healthcare spending, rising incidence of diseases, and improving research facilities.North America Diagnostics Enzyme Market Report:
North America holds a significant market position, with the Diagnostics Enzyme market valued at $2.07 billion in 2023, likely reaching $3.83 billion by 2033, supported by advanced healthcare infrastructure, increased R&D investments, and a high prevalence of chronic diseases.South America Diagnostics Enzyme Market Report:
The South American Diagnostics Enzyme market is projected to expand from $0.30 billion in 2023 to $0.55 billion by 2033, influenced by the growing focus on health diagnostics and access to modern healthcare facilities.Middle East & Africa Diagnostics Enzyme Market Report:
The Middle East and Africa are expected to see growth in the Diagnostics Enzyme market from $0.59 billion in 2023 to $1.09 billion by 2033, fueled by improvements in healthcare access and rising awareness about preventive healthcare.Tell us your focus area and get a customized research report.
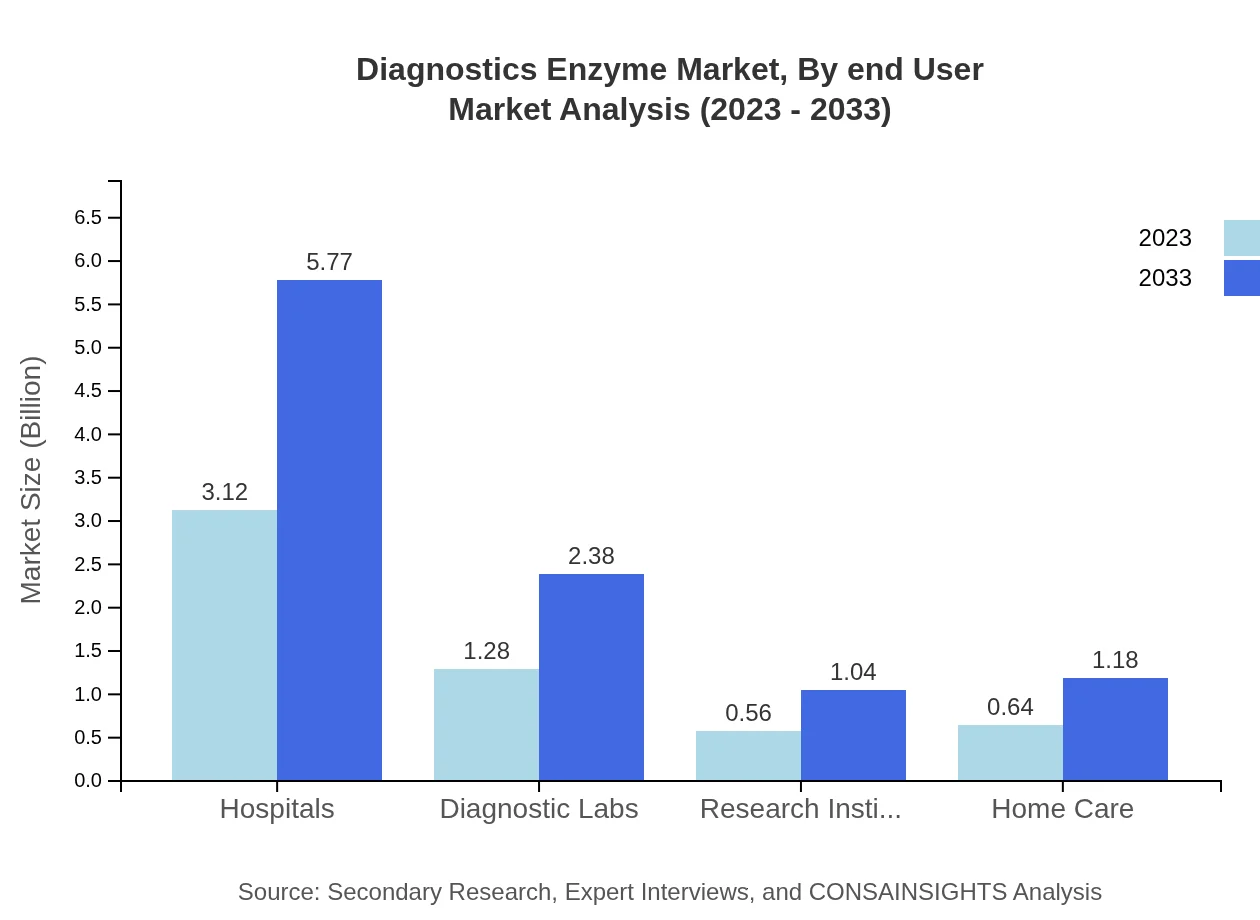
Diagnostics Enzyme Market Analysis By End User
Across end-users, hospitals dominate the Diagnostics Enzyme market with a share of 55.63% in 2023, rising to 55.63% in 2033. Diagnostic labs and research institutes represent 22.94% and 10.01% shares, respectively, underscoring their significant roles in diagnostics. Home care settings expand from 11.42% share as patients increasingly prefer at-home diagnostic solutions.
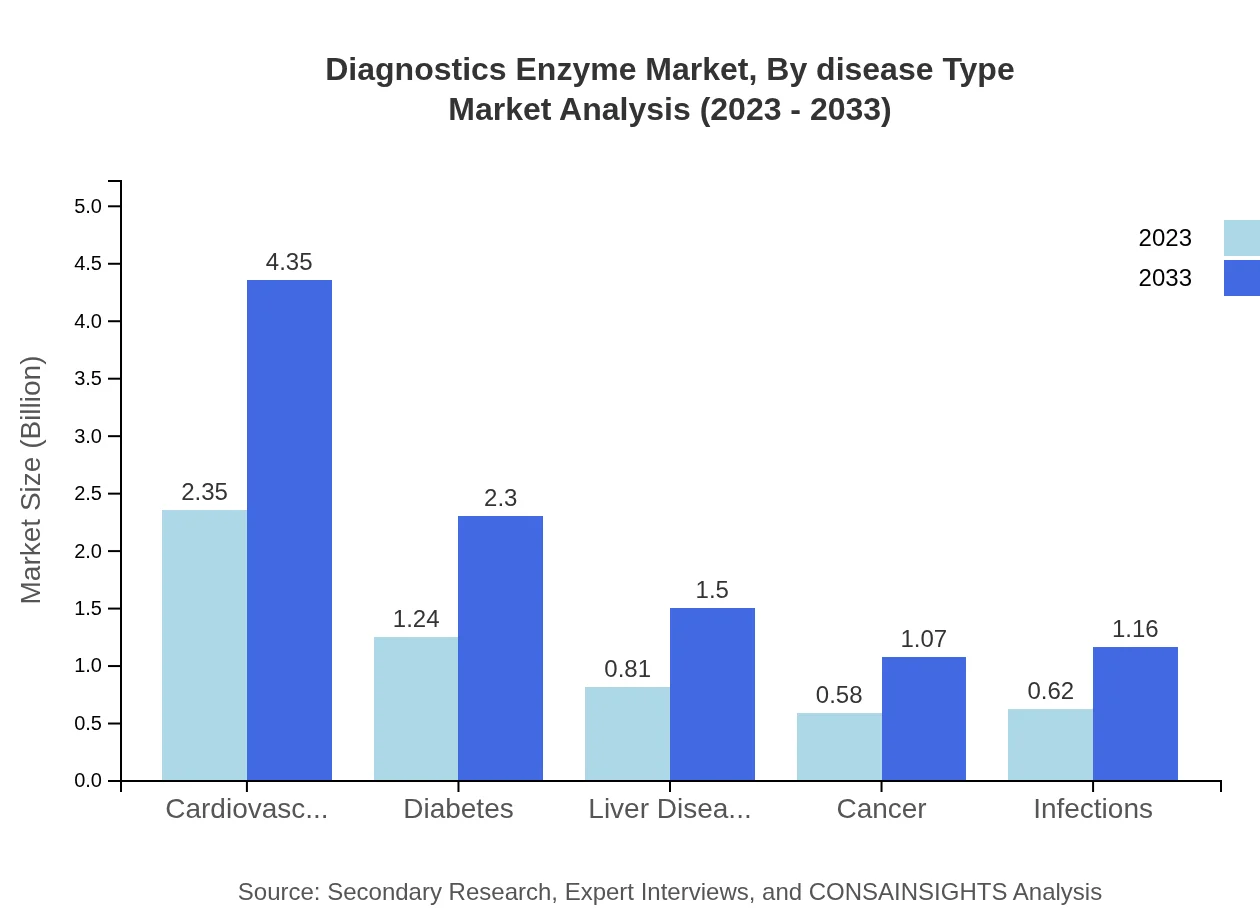
Diagnostics Enzyme Market Analysis By Disease Type
In terms of diseases, cardiovascular diseases constitute the largest segment, with a market size of $2.35 billion in 2023, expected to reach $4.35 billion by 2033. Diabetes follows with $1.24 billion, growing to $2.30 billion. Other segments include liver diseases and cancer, highlighting the importance of enzyme diagnostics in various medical conditions.
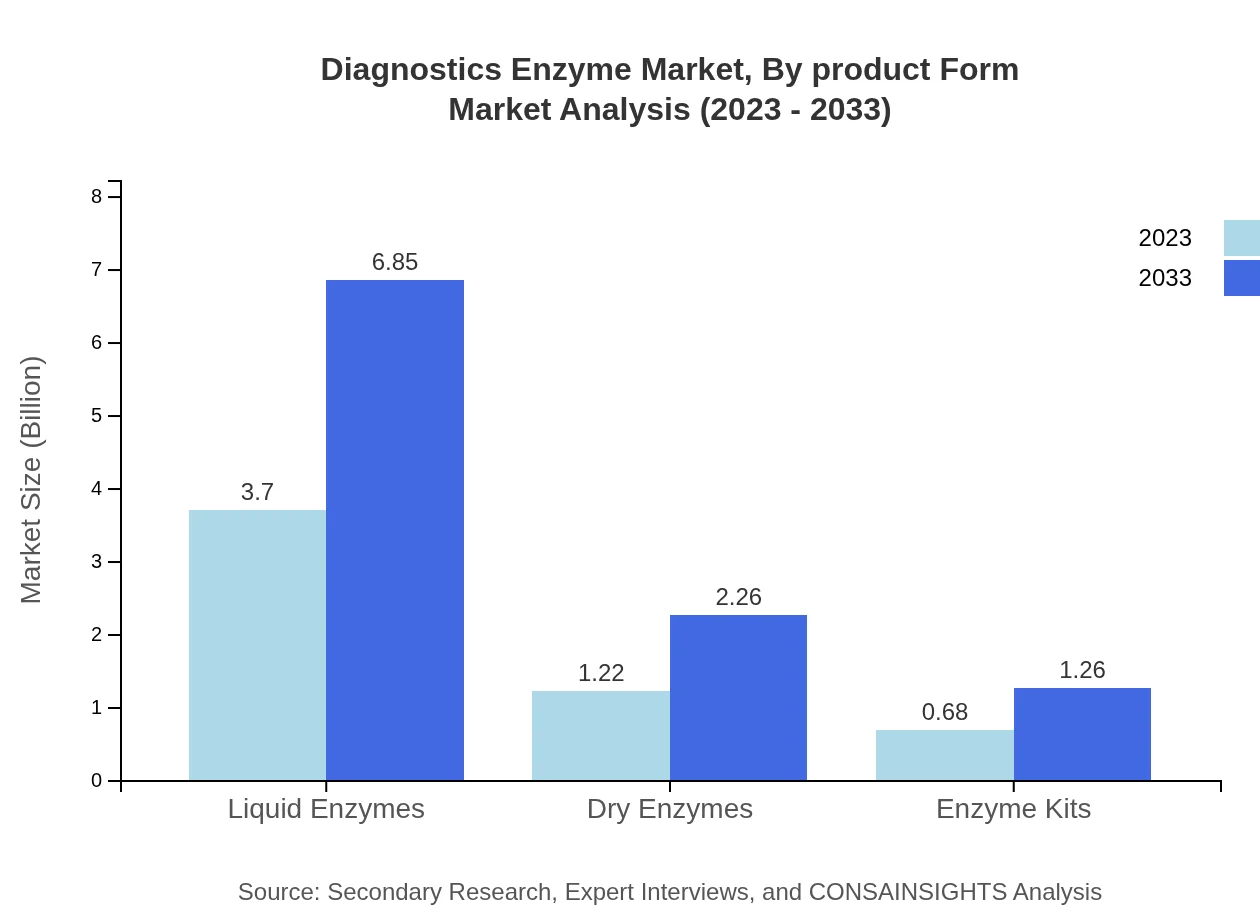
Diagnostics Enzyme Market Analysis By Product Form
Liquid enzymes dominate the market with a 66.06% share in 2023 and forecasted growth trajectory to $6.85 billion by 2033. Dry enzymes and enzyme kits are also significant, with shares of 21.76% and 12.18%, respectively, reflecting diverse product developments that cater to different diagnostic needs.
Diagnostics Enzyme Market Analysis By Enzymatic Action
Hydrolases command a substantial market presence, representing 66.06% of the overall market in 2023, expected to grow significantly. Meanwhile, oxidoreductases and transferases serve critical functions in diagnostic applications, making them essential for market dynamics.
Diagnostics Enzyme Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Diagnostics Enzyme Industry
Roche Diagnostics:
A leader in diagnostics that develops innovative diagnostic solutions, including enzyme-based assays for various diseases.Abbott Laboratories:
Known for its cutting-edge diagnostic technologies, Abbott's enzymes play a crucial role in medical testing and disease management.Thermo Fisher Scientific:
This company specializes in biotechnological products including enzymes, dedicated to improving diagnostic and therapeutic outcomes.Danaher Corporation:
Focused on diagnostic technologies, Danaher's innovations in enzyme assays contribute significantly to healthcare solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of diagnostics Enzyme?
The global diagnostics enzyme market is valued at approximately $5.6 billion in 2023 and is projected to grow at a CAGR of 6.2%, enhancing diagnostic capabilities across various sectors until 2033.
What are the key market players or companies in the diagnostics Enzyme industry?
Key players in the diagnostics enzyme market include companies like Thermo Fisher Scientific, Roche, and Siemens Healthineers, which provide a range of enzyme products and contribute significantly to market advancements.
What are the primary factors driving the growth in the diagnostics enzyme industry?
Growth in the diagnostics enzyme market is driven by rising prevalence of chronic diseases, advancements in diagnostic technologies, increasing demand for point-of-care testing, and ongoing research in enzyme applications.
Which region is the fastest Growing in the diagnostics enzyme?
The Asia Pacific region is the fastest-growing market for diagnostics enzymes, projected to rise from $1.17 billion in 2023 to $2.17 billion by 2033, reflecting a strong demand for innovative healthcare solutions.
Does ConsaInsights provide customized market report data for the diagnostics enzyme industry?
Yes, ConsaInsights offers customized market report data for the diagnostics enzyme industry, enabling clients to access tailored insights that meet specific business needs and strategic objectives.
What deliverables can I expect from this diagnostics Enzyme market research project?
Expected deliverables from the diagnostics enzyme market research project include comprehensive reports, data analysis, market forecasts, competitive analysis, and strategic recommendations approximately covering a 10-year outlook.
What are the market trends of diagnostics enzyme?
Current trends in the diagnostics enzyme market include increasing use of liquid enzymes, advancements in enzymatic assays, and the growing importance of rapid diagnostic tests, reflecting innovation in enzyme applications.