Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report
Published Date: 31 January 2026 | Report Code: dipeptide-peptidase-4-dpp-4-inhibitors
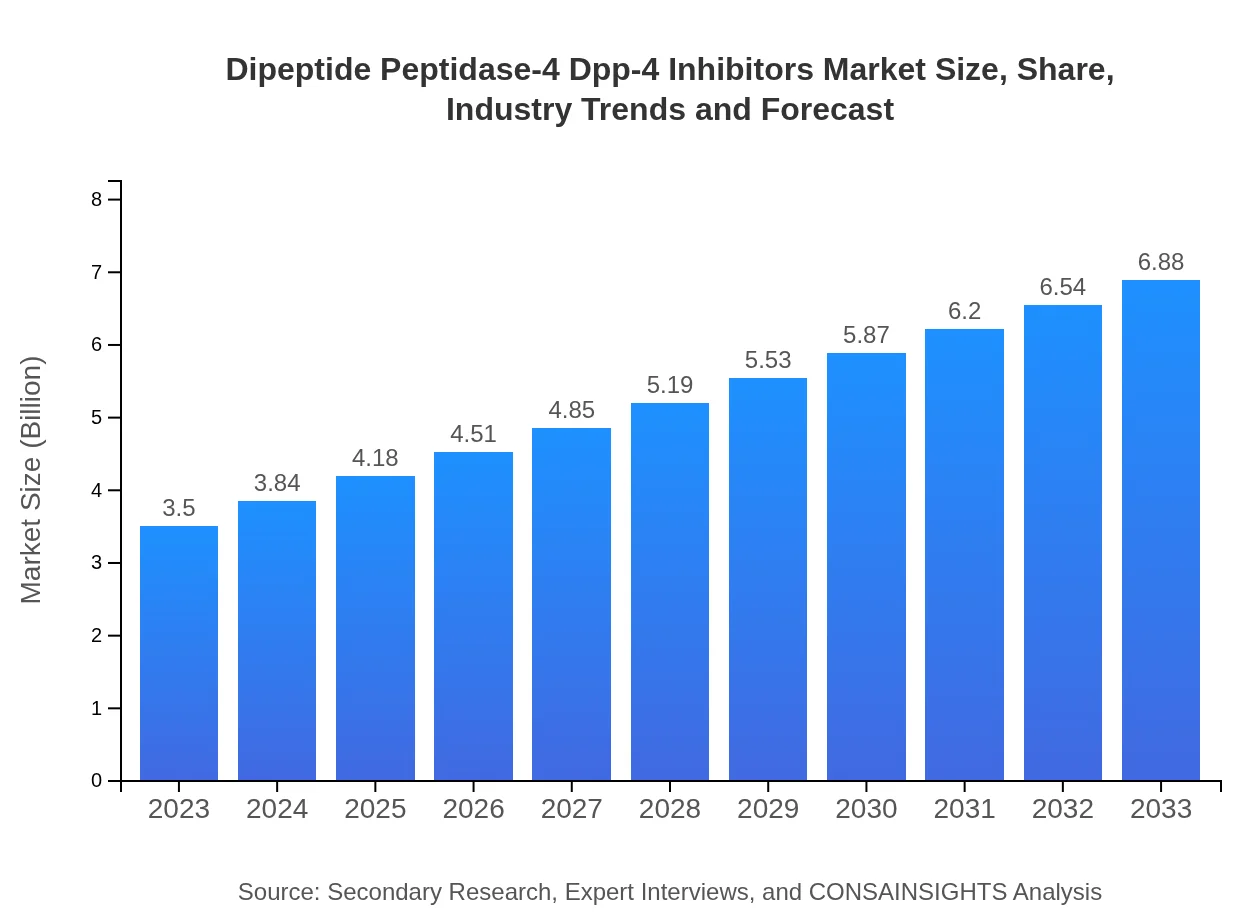
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Dipeptide Peptidase-4 (DPP-4) inhibitors market from 2023 to 2033, including insights on market size, growth forecasts, regional analysis, technology trends, product performance, and leading market players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Merck & Co., Boehringer Ingelheim, AstraZeneca, Sanofi |
| Last Modified Date | 31 January 2026 |
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Overview
Customize Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report market research report
- ✔ Get in-depth analysis of Dipeptide Peptidase-4 Dpp-4 Inhibitors market size, growth, and forecasts.
- ✔ Understand Dipeptide Peptidase-4 Dpp-4 Inhibitors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Dipeptide Peptidase-4 Dpp-4 Inhibitors
What is the Market Size & CAGR of Dipeptide Peptidase-4 Inhibitors market in 2023?
Dipeptide Peptidase-4 Dpp-4 Inhibitors Industry Analysis
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis Report by Region
Europe Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report:
The European market is projected to increase from USD 0.91 billion in 2023 to USD 1.79 billion in 2033, as countries within the region enhance diabetes care standards and innovative treatments become more available.Asia Pacific Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report:
The Asia Pacific region is witnessing substantial growth in the DPP-4 inhibitors market, projected to rise from USD 0.73 billion in 2023 to USD 1.44 billion in 2033, driven by increasing diabetes prevalence and healthcare infrastructure improvement.North America Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report:
North America, particularly the United States, leads the market with expectations to grow from USD 1.19 billion in 2023 to USD 2.34 billion by 2033, supported by advanced healthcare systems and a high rate of diabetes diagnoses.South America Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report:
In South America, the market is expected to grow from USD 0.25 billion in 2023 to USD 0.49 billion in 2033. Factors contributing to this growth include rising healthcare investments and growing awareness regarding diabetes management.Middle East & Africa Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Report:
The Middle East and Africa market is set to grow from USD 0.42 billion in 2023 to USD 0.82 billion by 2033, propelled by rising diabetes cases and initiatives to improve healthcare accessibility.Tell us your focus area and get a customized research report.
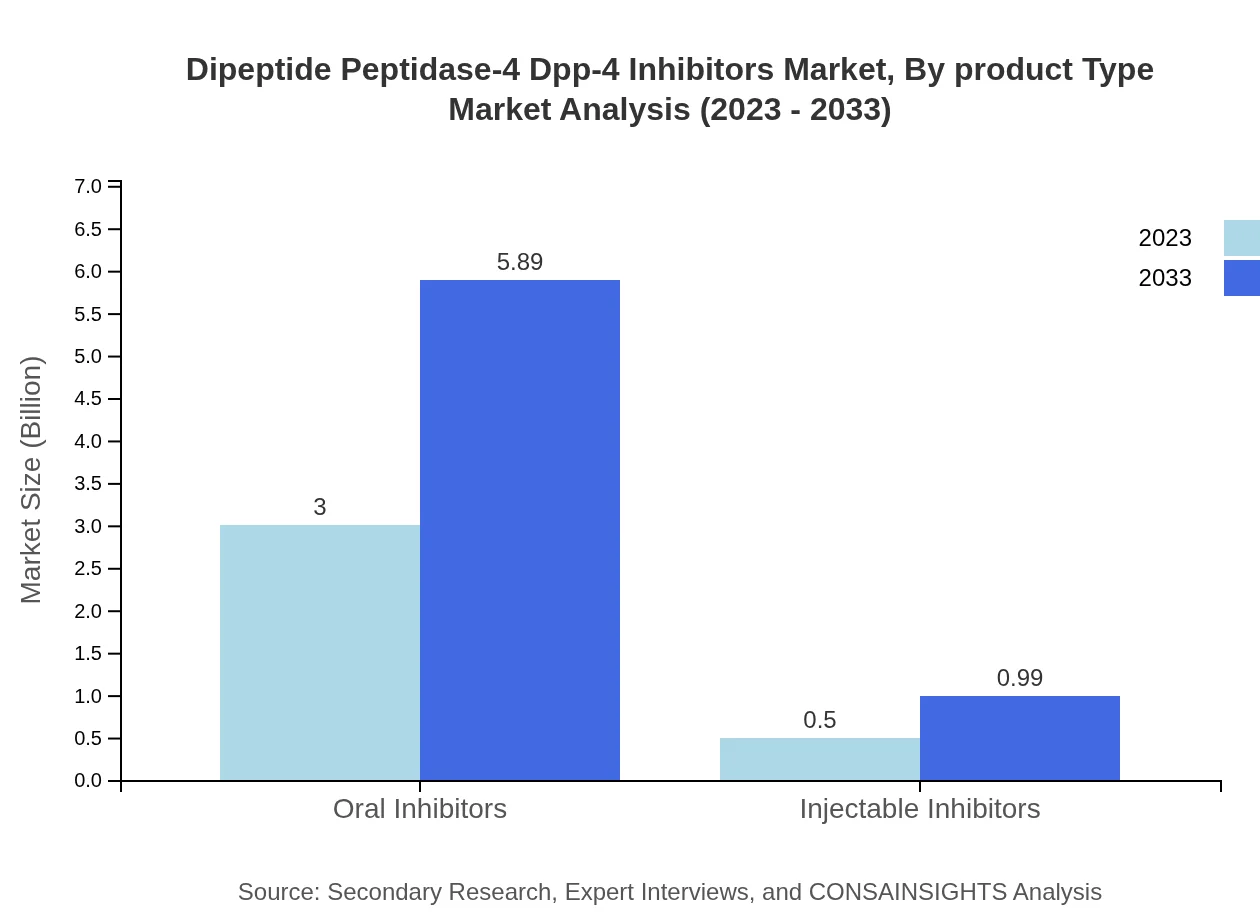
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis By Product Type
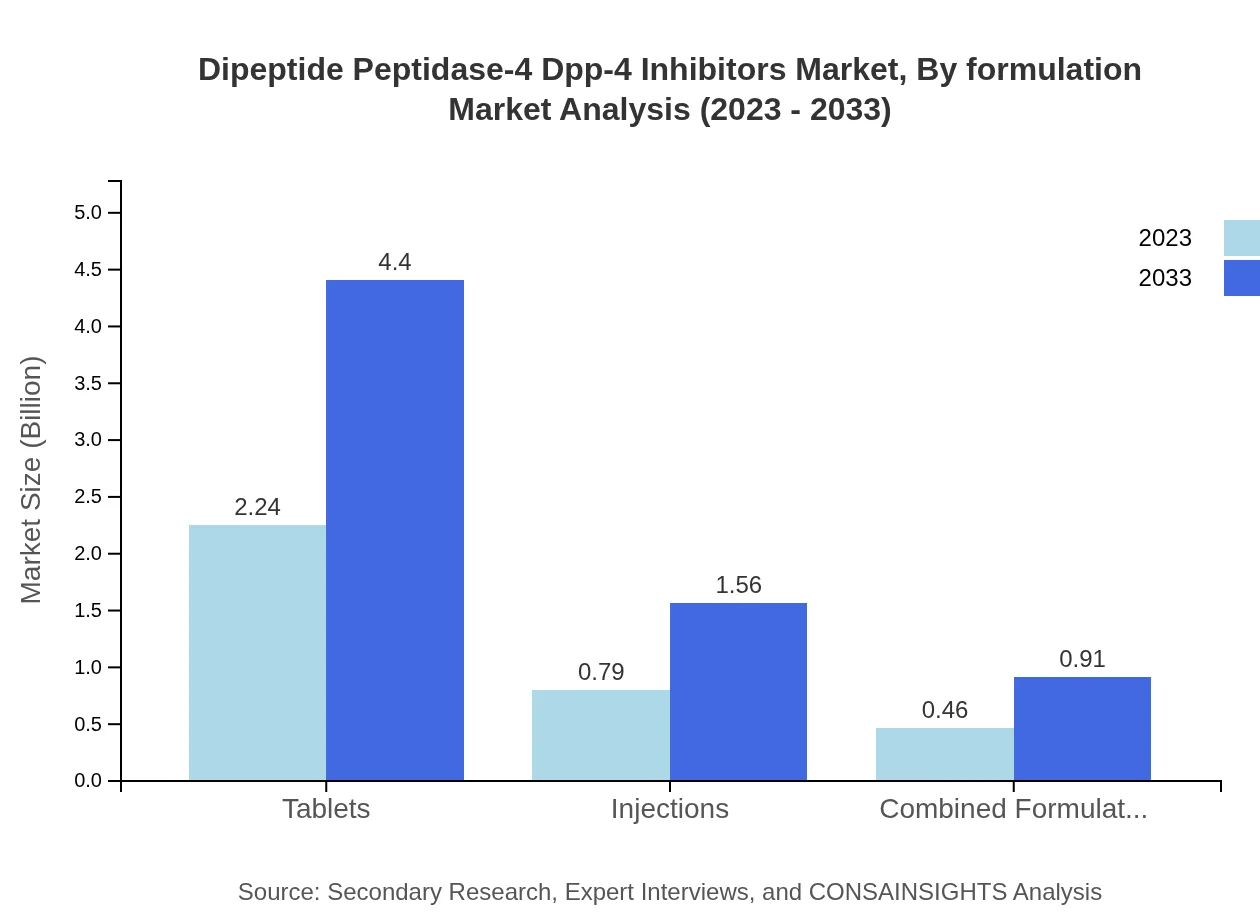
In 2023, the oral inhibitors segment leads the market with a share of 85.65%, valued at USD 3.00 billion, and is expected to grow to USD 5.89 billion by 2033. Tablets also show significant growth, anticipated to grow from USD 2.24 billion to USD 4.40 billion over the same period. Injectables and combined formulations are gaining traction but represent smaller shares in the overall market.
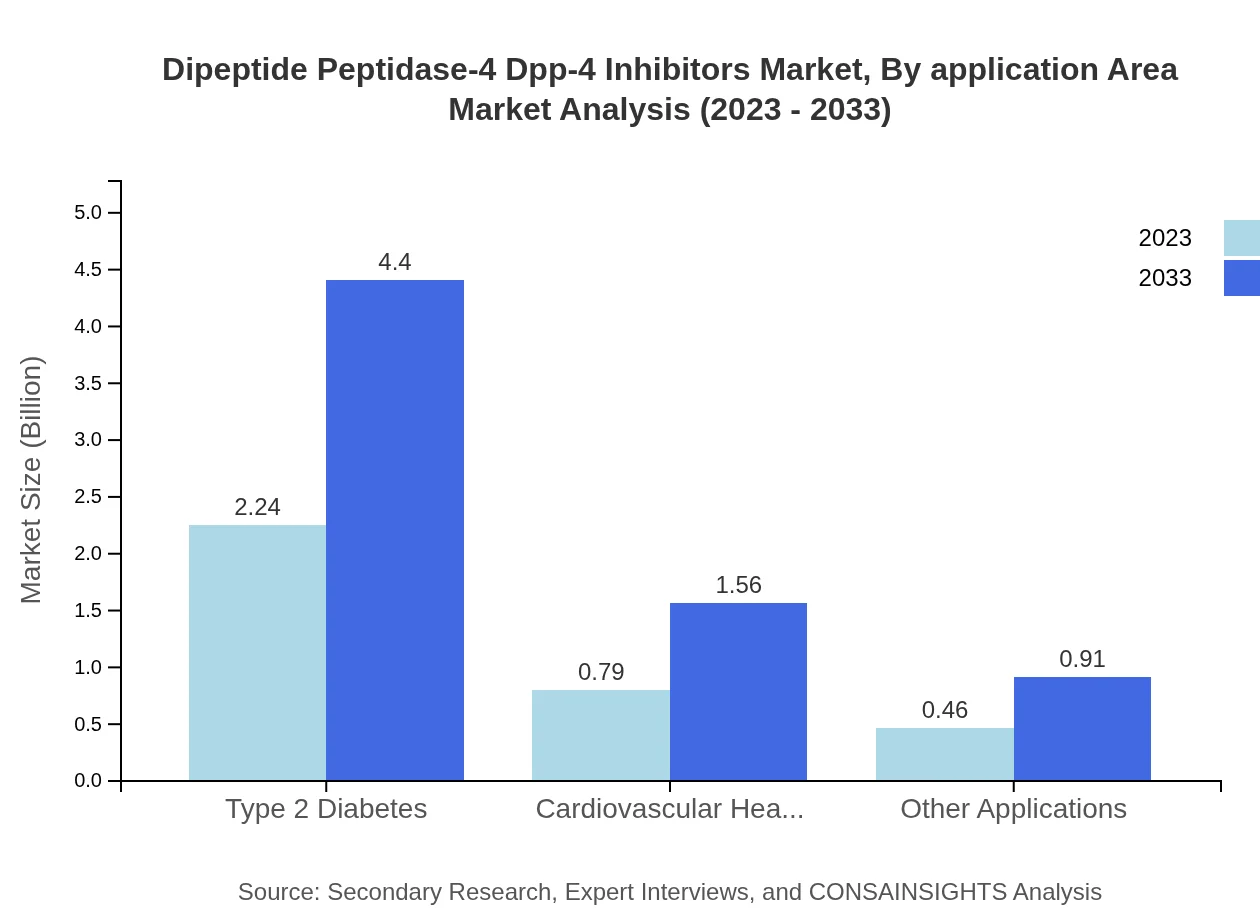
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis By Application Area
Type 2 diabetes is the main application area, with a market size of USD 2.24 billion in 2023, expected to reach USD 4.40 billion by 2033. Cardiovascular health and other applications also hold significant potential, with growth driven by rising health concerns and the effectiveness of DPP-4 inhibitors in managing comorbid conditions.
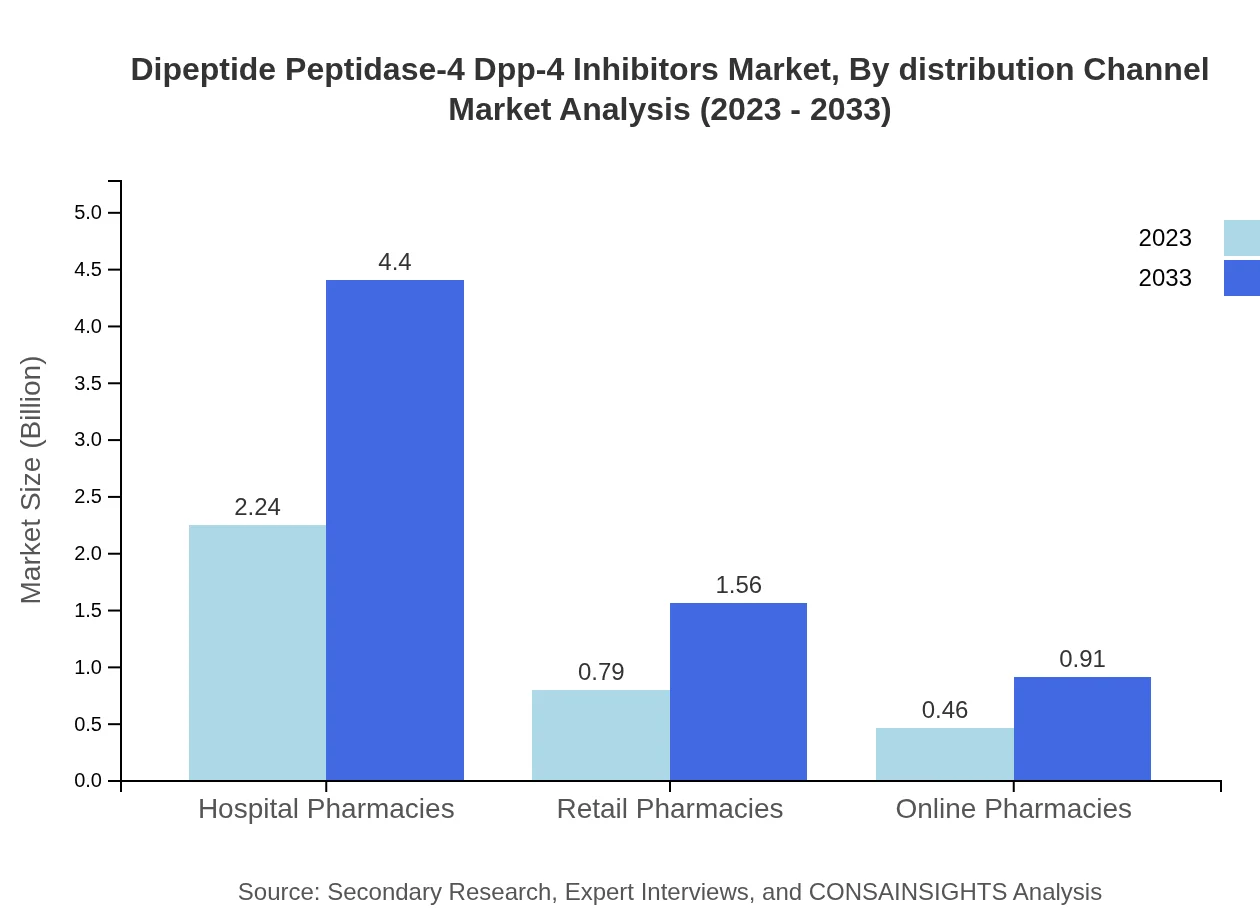
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis By Distribution Channel
Hospital pharmacies dominated distribution channels with a market share of 64.03% in 2023, valued at USD 2.24 billion, expected to grow to USD 4.40 billion by 2033. Retail and online pharmacies also contribute significantly, focusing on accessibility and convenience for patients.
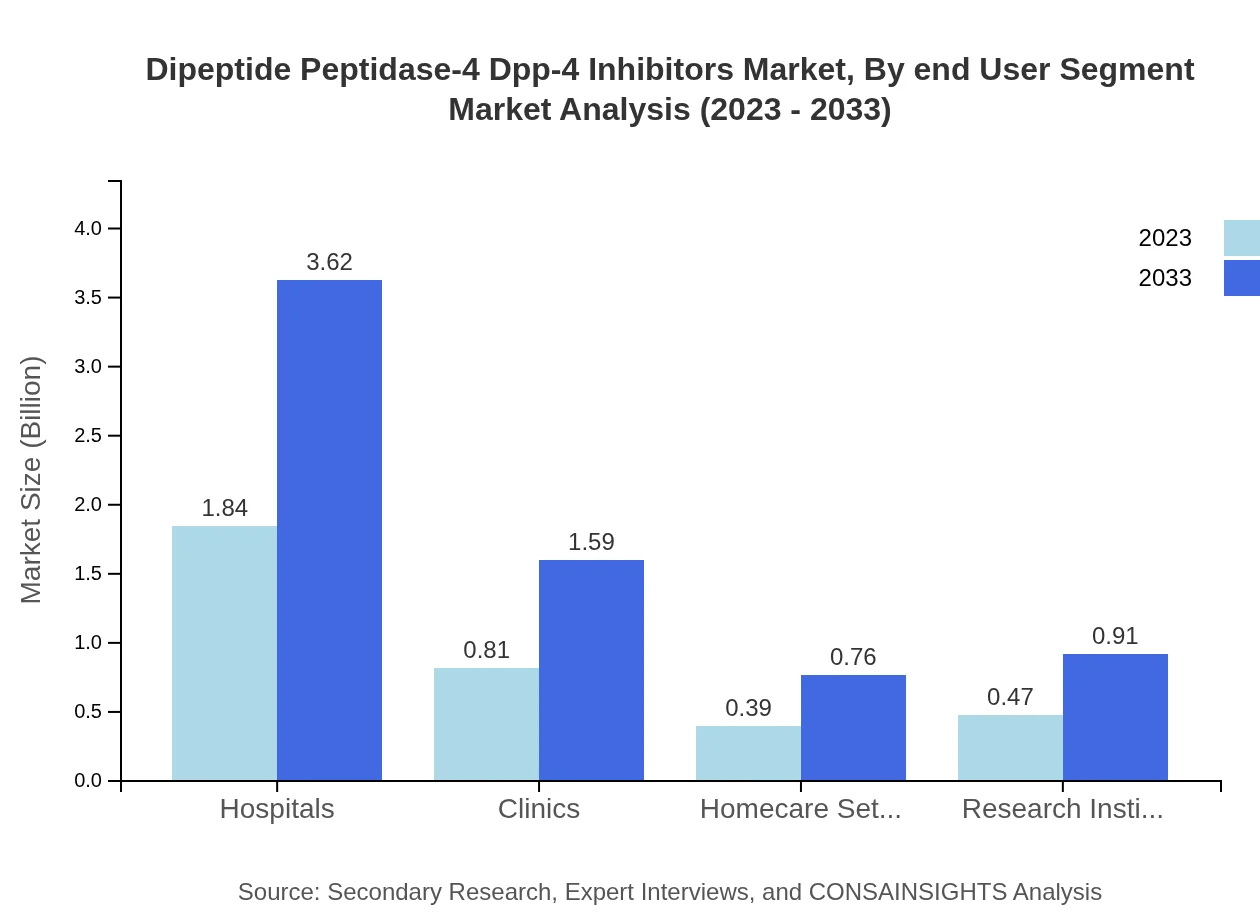
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis By End User Segment
Hospitals are the primary end-users, expected to grow from USD 1.84 billion in 2023 to USD 3.62 billion in 2033, commanding a 52.56% market share. Clinics and homecare settings also play important roles, reflecting trends toward outpatient care and chronic disease management.
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Analysis By Formulation
The formulation segment is split predominantly between oral and injectable methods, with oral formulations accounting for the larger share. As convenience and patient preference play crucial roles in medication adherence, innovations in formulations continue to be a key focus area for market players.
Dipeptide Peptidase-4 Dpp-4 Inhibitors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Dipeptide Peptidase-4 Dpp-4 Inhibitors Industry
Merck & Co.:
A leading pharmaceutical company known for its pioneering drug Januvia (sitagliptin), which significantly contributes to the DPP-4 inhibitors market.Boehringer Ingelheim:
Recognized for developing Trajenta (linagliptin), Boehringer Ingelheim is at the forefront of DPP-4 inhibitor research and development.AstraZeneca:
AstraZeneca manufactures Bydureon, expanding the DPP-4 inhibitor offering and focusing on innovative diabetes therapies.Sanofi:
Sanofi is engaged in the diabetes market with drugs like Lyxumia, enhancing therapeutic choices for patients.We're grateful to work with incredible clients.









FAQs
What is the market size of dipeptide Peptidase-4 Dpp-4 Inhibitors?
The global market size for dipeptide-peptidase-4 (DPP-4) inhibitors is currently valued at approximately $3.5 billion. The market is projected to experience a compound annual growth rate (CAGR) of 6.8% from 2023 to 2033.
What are the key market players or companies in this dipeptide Peptidase-4 Dpp-4 Inhibitors industry?
Key players in the DPP-4 inhibitors market include major companies such as Merck & Co., Bristol-Myers Squibb, and Novo Nordisk, which dominate with their innovative products and strong market presence.
What are the primary factors driving the growth in the dipeptide Peptidase-4 Dpp-4 Inhibitors industry?
Growth in the DPP-4 inhibitors market is driven by the rising prevalence of type 2 diabetes, increasing awareness about diabetes management, and advancements in drug development that enhance the efficacy and safety of treatments.
Which region is the fastest Growing in the dipeptide Peptidase-4 Dpp-4 Inhibitors?
The Asia-Pacific region is identified as the fastest-growing market for DPP-4 inhibitors, expected to grow from $0.73 billion in 2023 to $1.44 billion by 2033, reflecting a significant increase in diabetic patient populations.
Does ConsaInsights provide customized market report data for the dipeptide Peptidase-4 Dpp-4 Inhibitors industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients within the DPP-4 inhibitors industry, ensuring focused insights for strategic decision-making.
What deliverables can I expect from this dipeptide Peptidase-4 Dpp-4 Inhibitors market research project?
Clients can expect comprehensive reports, including detailed market analysis, trends, competitive landscape, segmentation data, and forecasts to guide investment and strategic planning in the DPP-4 inhibitors market.
What are the market trends of dipeptide Peptidase-4 Dpp-4 Inhibitors?
Emerging trends in the DPP-4 inhibitors market include the shift towards combination therapies, increasing demand for oral formulations, and ongoing research to develop more advanced and effective treatments for type 2 diabetes.