Divalproex Sodium Market Report
Published Date: 31 January 2026 | Report Code: divalproex-sodium
Divalproex Sodium Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Divalproex Sodium market, including market size, trends, segmentation, and forecasts for 2023 to 2033. Insights into regional performance, leading companies, and technological advancements are also covered, aiding stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
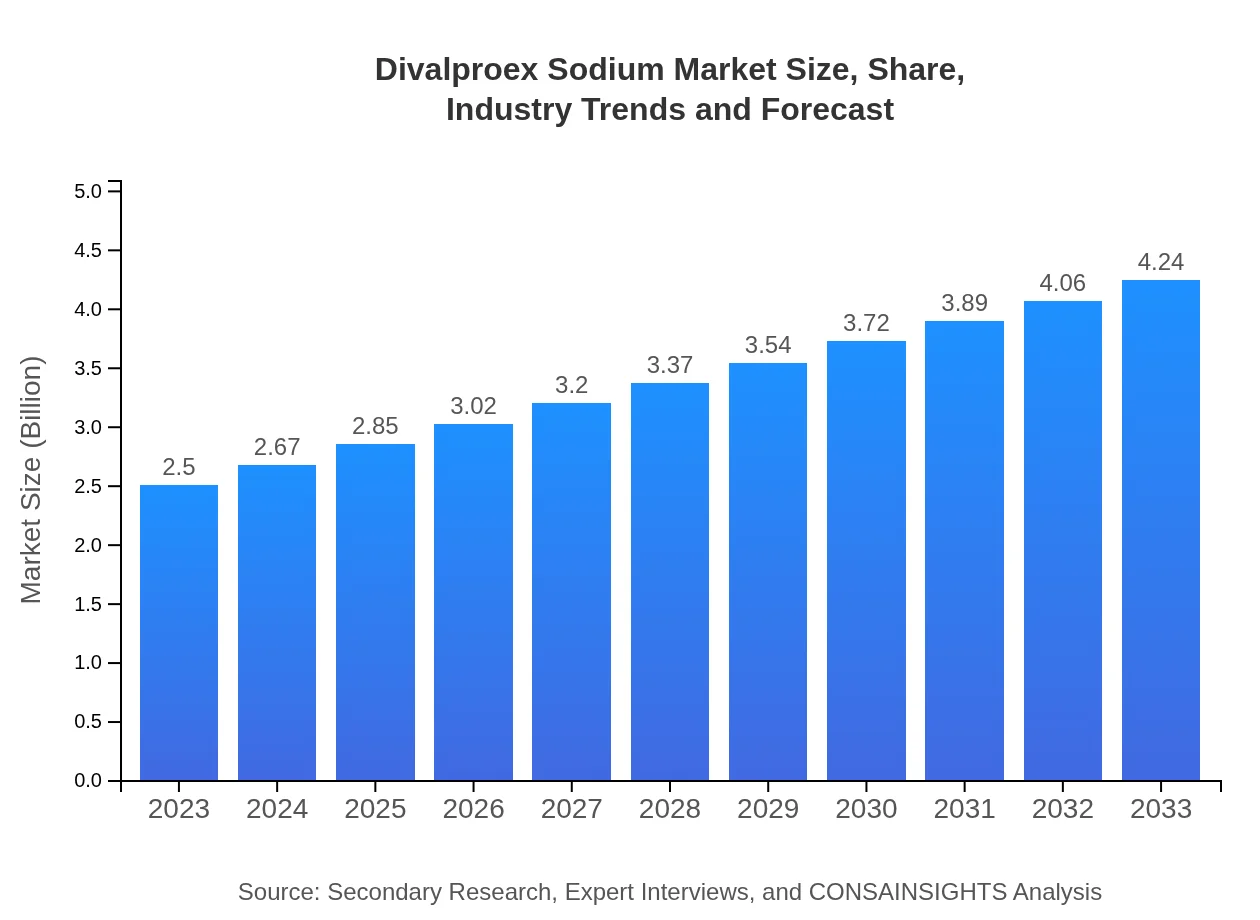
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.3% |
| 2033 Market Size | $4.24 Billion |
| Top Companies | AbbVie Inc., Teva Pharmaceutical Industries Ltd., Mylan N.V., Pfizer Inc. |
| Last Modified Date | 31 January 2026 |
Divalproex Sodium Market Overview
Customize Divalproex Sodium Market Report market research report
- ✔ Get in-depth analysis of Divalproex Sodium market size, growth, and forecasts.
- ✔ Understand Divalproex Sodium's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Divalproex Sodium
What is the Market Size & CAGR of Divalproex Sodium market in 2023?
Divalproex Sodium Industry Analysis
Divalproex Sodium Market Segmentation and Scope
Tell us your focus area and get a customized research report.
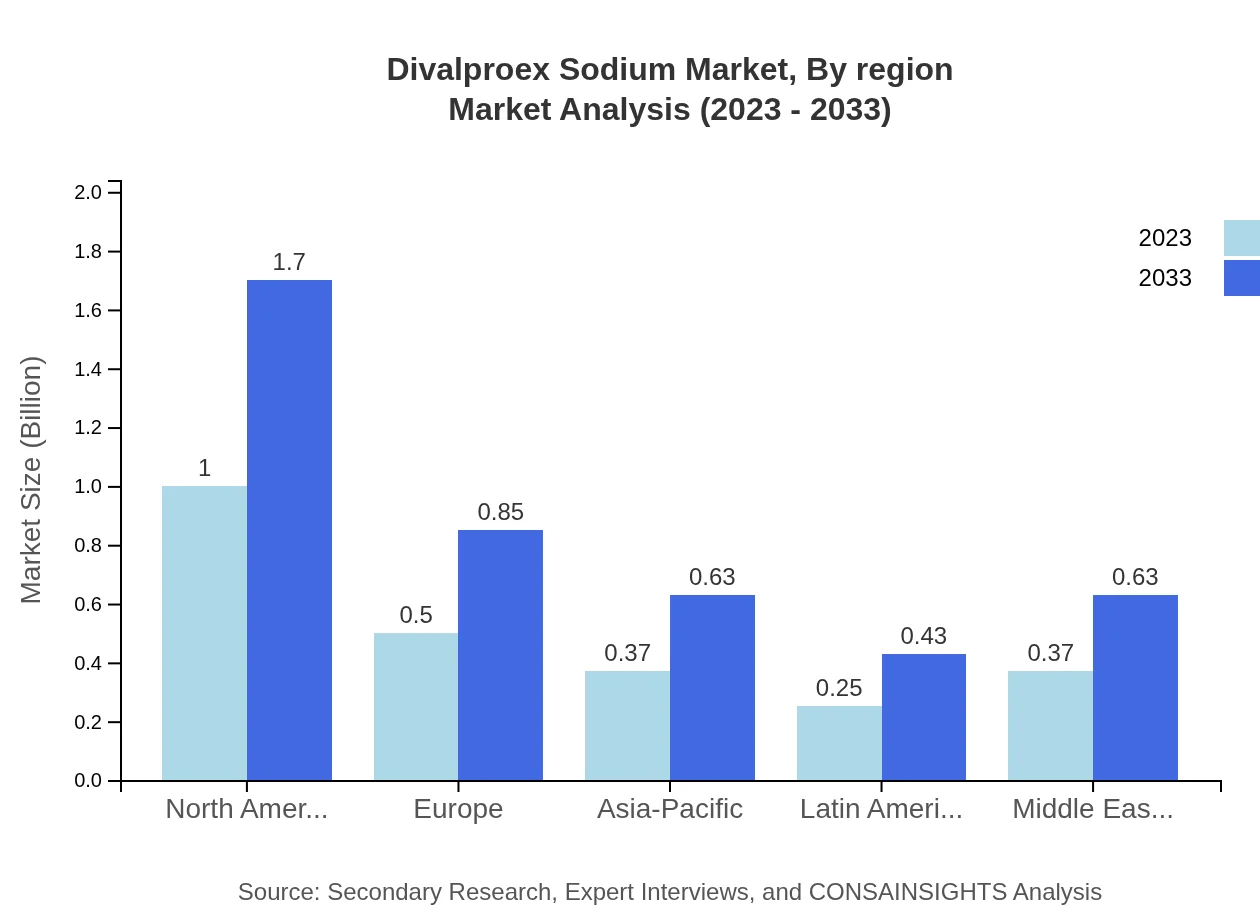
Divalproex Sodium Market Analysis Report by Region
Europe Divalproex Sodium Market Report:
In Europe, the market is set to grow from $0.71 billion in 2023 to $1.20 billion by 2033. The region exhibits strong regulatory frameworks that encourage innovation. Growing aging populations and increased prevalence of chronic conditions further contribute to market expansion in countries like Germany and France.Asia Pacific Divalproex Sodium Market Report:
In the Asia Pacific region, the Divalproex Sodium market is projected to grow from $0.48 billion in 2023 to $0.82 billion in 2033. The increase in healthcare infrastructure investments and rising awareness of mental health issues are driving this growth. Emerging markets like India and China are central to this expansion, supported by government initiatives and increased demand for pharmacological treatments.North America Divalproex Sodium Market Report:
North America holds a prominent position in the Divalproex Sodium market, expected to expand from $0.92 billion in 2023 to $1.57 billion in 2033. The region benefits from advanced healthcare systems, high awareness of mental health disorders, and ongoing research initiatives, establishing North America as a leading player in market development.South America Divalproex Sodium Market Report:
The market in South America is anticipated to grow from $0.24 billion in 2023 to $0.40 billion by 2033, propelled by an increase in healthcare accessibility and investments in pharmaceutical R&D. Brazil and Argentina are key players in this growth, focusing on improving healthcare outcomes.Middle East & Africa Divalproex Sodium Market Report:
The Middle East and Africa market is projected to grow from $0.15 billion in 2023 to $0.25 billion by 2033. Factors such as improving healthcare access and an increasing focus on mental health contribute to growth. However, challenges such as limited resources and varying regulatory standards may hinder rapid market development in certain areas.Tell us your focus area and get a customized research report.
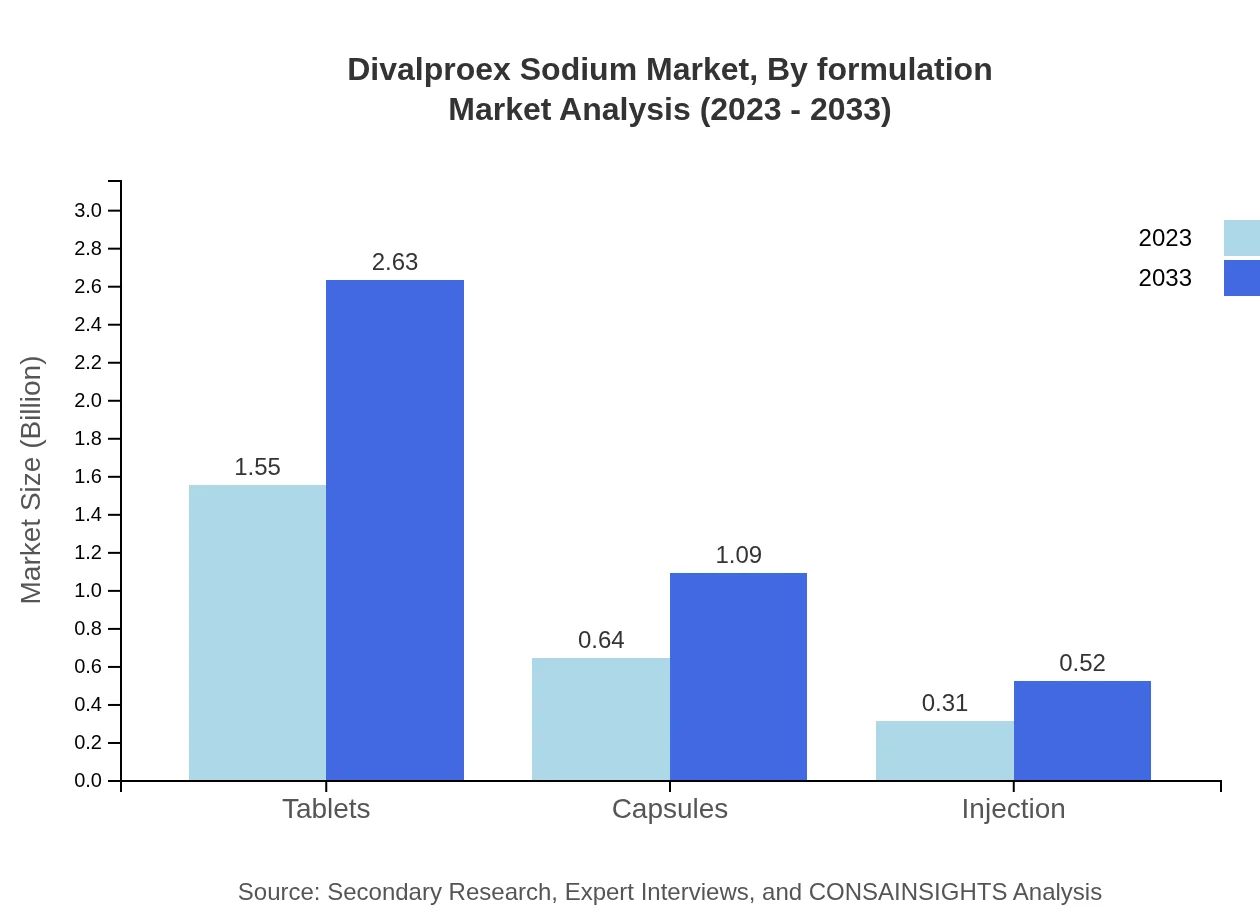
Divalproex Sodium Market Analysis By Formulation
The Divalproex Sodium market by formulation includes Tablets, Capsules, and Injections. Tablets lead the market, accounting for 62.1% of the total market share in 2023, projected to grow significantly to meet the increasing demand in the upcoming decade. Capsules and injections, while smaller segments, also show growth potential due to their targeted usage in specific patient populations, thus maintaining their relevance in the overall market.
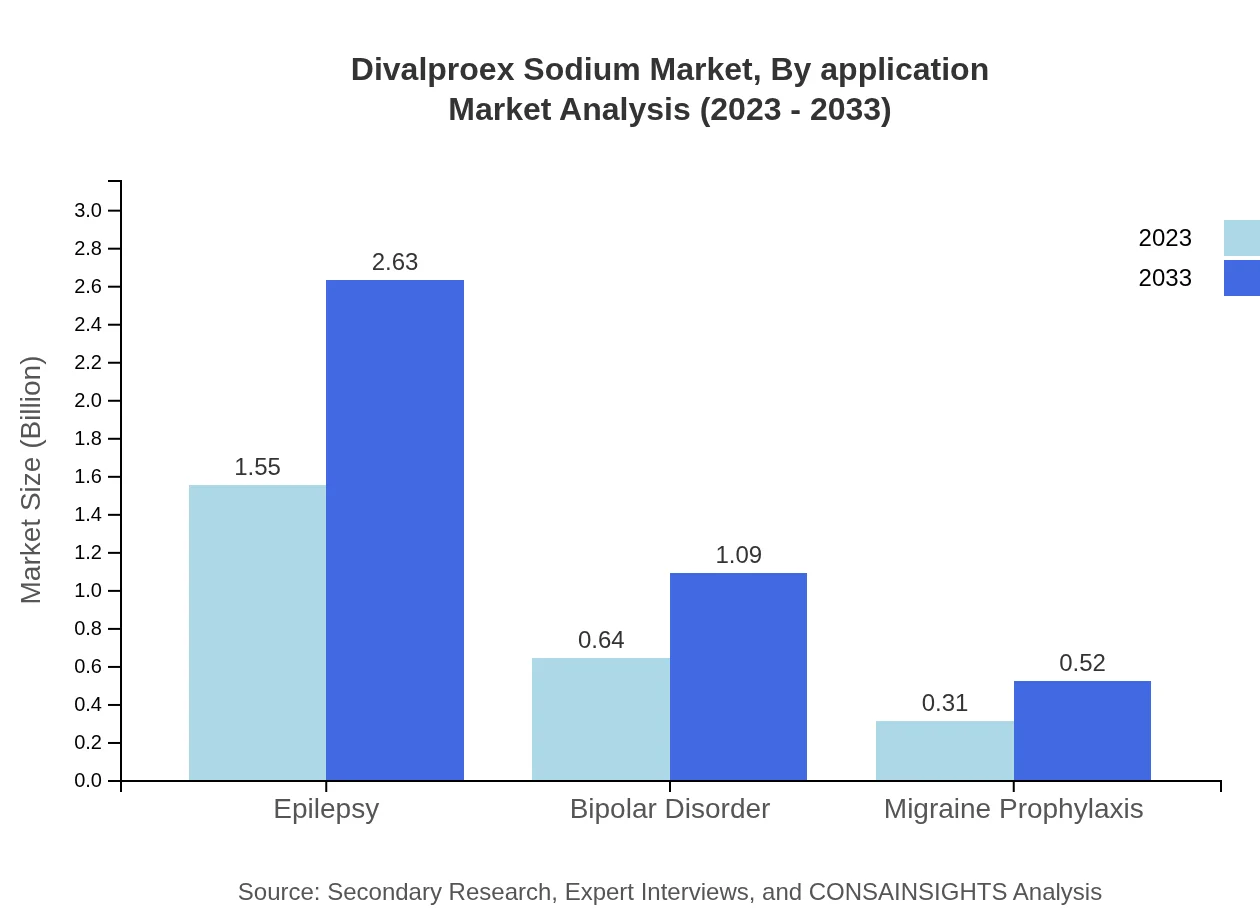
Divalproex Sodium Market Analysis By Application
The application segments include Epilepsy, Bipolar Disorder, and Migraine Prophylaxis. The Epilepsy segment accounts for the majority share at 62.1% in 2023, driven by rising diagnosis rates. Bipolar disorder treatment represents a substantial 25.69% share, with an expected increase as awareness grows, while migraine prophylaxis takes 12.21%, reflecting a growing recognition of its therapeutic benefits.
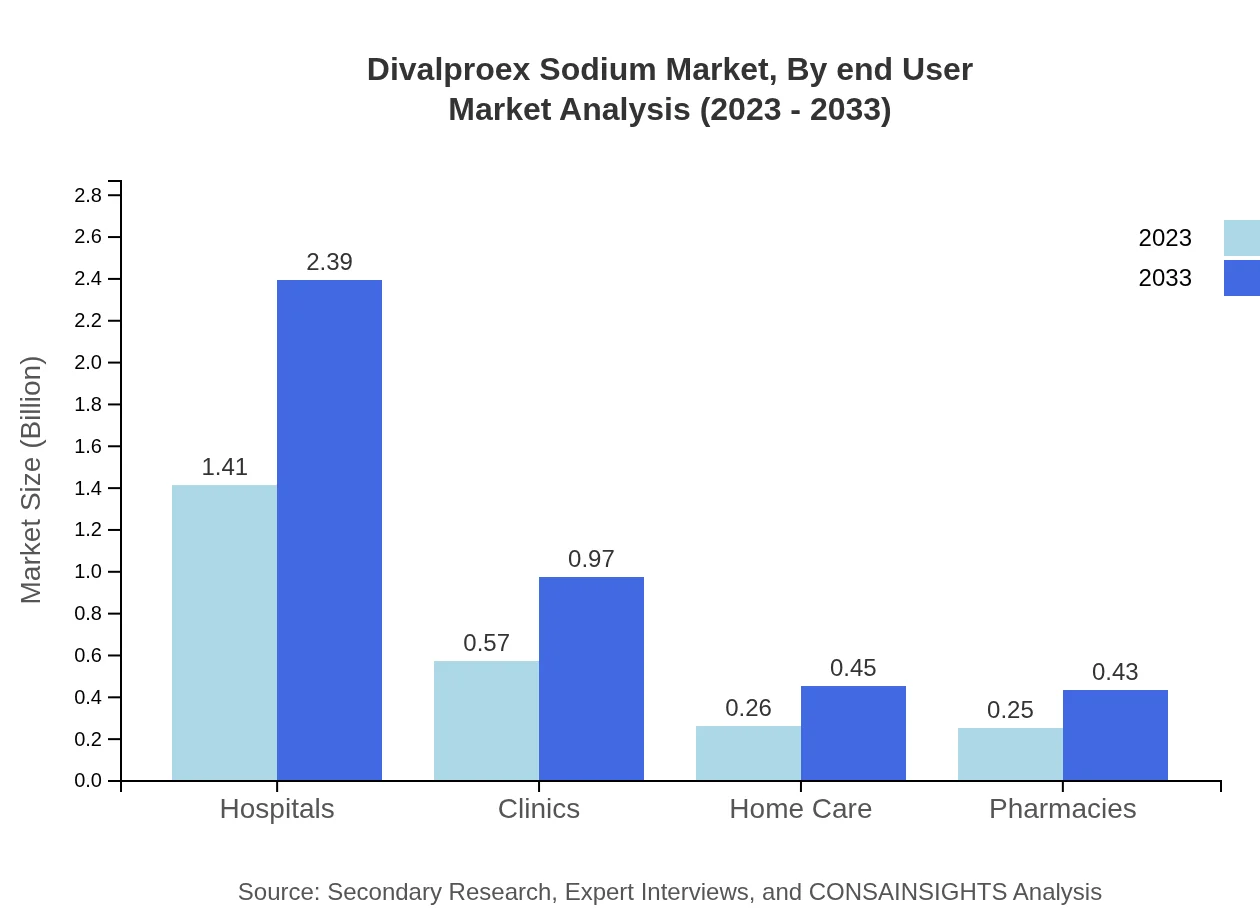
Divalproex Sodium Market Analysis By End User
The healthcare setting segments include Hospitals, Clinics, and Home Care. Hospitals hold a 56.5% share in 2023, owing to the high demand for inpatient treatments. Clinics follow with 22.95%, catering to outpatient needs, while Home Care represents a growing segment as more patients seek treatment in familiar environments.
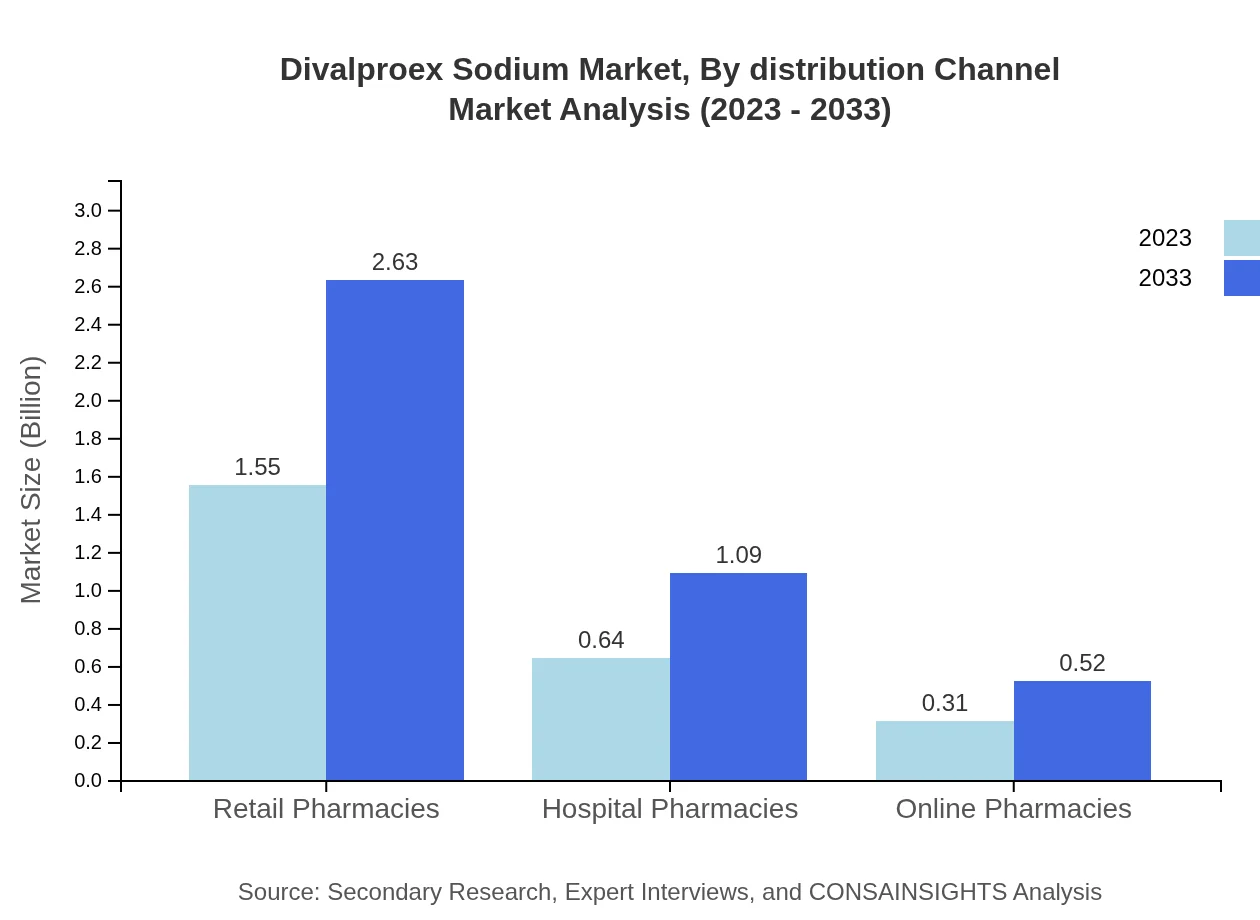
Divalproex Sodium Market Analysis By Distribution Channel
The distribution channels include Retail Pharmacies, Hospital Pharmacies, and Online Pharmacies. Retail Pharmacies dominate with 62.1% in 2023, reflecting patient preference for easy access to medications. Hospital Pharmacies account for 25.69%, serving inpatient needs, while Online Pharmacies represent 12.21%, capitalizing on the growing e-commerce trend in health products.
Divalproex Sodium Market Analysis By Region
Different regions exhibit unique trends based on healthcare infrastructure and market dynamics. North America leads in market size, while emerging markets in Asia-Pacific present significant opportunities for growth. Europe showcases a commitment to innovative therapies, and the Middle East and Africa are gradually evolving as healthcare prioritizes mental health.
Divalproex Sodium Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Divalproex Sodium Industry
AbbVie Inc.:
A leading pharmaceutical company focusing on innovative treatments across various therapeutic areas, including neurology and immunology.Teva Pharmaceutical Industries Ltd.:
A global leader in generic pharmaceuticals, Teva provides affordable access to vital medications, including Divalproex Sodium formulations.Mylan N.V.:
A significant player in the generic drug sector, Mylan enhances patient access to therapies through a diverse portfolio, including Divalproex Sodium.Pfizer Inc.:
With a strong focus on research and development, Pfizer drives innovation in health sciences and plays a vital role in the Divalproex Sodium market.We're grateful to work with incredible clients.









FAQs
What is the market size of divalproex Sodium?
The market size of divalproex-sodium is approximately $2.5 billion in 2023, with an expected CAGR of 5.3% over the next decade, indicating robust growth and expanding opportunities for stakeholders in the pharmaceutical industry.
What are the key market players or companies in the divalproex Sodium industry?
Key players in the divalproex-sodium industry include major pharmaceutical companies involved in producing generic and brand name formulations. Competitive dynamics are driven by innovation and pricing strategies, alongside regulatory compliance and patent considerations which dictate market penetration.
What are the primary factors driving the growth in the divalproex Sodium industry?
Growth in the divalproex-sodium market is driven by increasing prevalence of epilepsy and bipolar disorder, advancement in drug formulations, healthcare awareness, and enhanced access to treatment options in various regions, empowering patients with extensive therapeutic choices.
Which region is the fastest Growing in the divalproex Sodium?
North America is currently the fastest-growing region in the divalproex-sodium market, projected to expand from $0.92 billion in 2023 to $1.57 billion by 2033, fueled by robust healthcare infrastructure and high demand for psychiatric medications.
Does ConsaInsights provide customized market report data for the divalproex Sodium industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the divalproex-sodium industry, ensuring comprehensive analysis and insights aligned with client requirements for effective decision-making.
What deliverables can I expect from this divalproex Sodium market research project?
Clients can expect detailed market research reports, comprehensive data analytics, trends and forecasts, competitive landscape analysis, and strategic recommendations to capture opportunities within the divalproex-sodium space.
What are the market trends of divalproex Sodium?
Market trends in divalproex-sodium indicate a shift towards personalized medicine, increasing focus on mental health awareness, growing generic competition, and emerging formulations that address specific conditions creating new avenues for market growth.