Drug Eluting Balloon Market Report
Published Date: 31 January 2026 | Report Code: drug-eluting-balloon
Drug Eluting Balloon Market Size, Share, Industry Trends and Forecast to 2033
This report provides a detailed analysis of the Drug Eluting Balloon market from 2023 to 2033, including insights on market size, growth trends, regional analysis, and key players within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
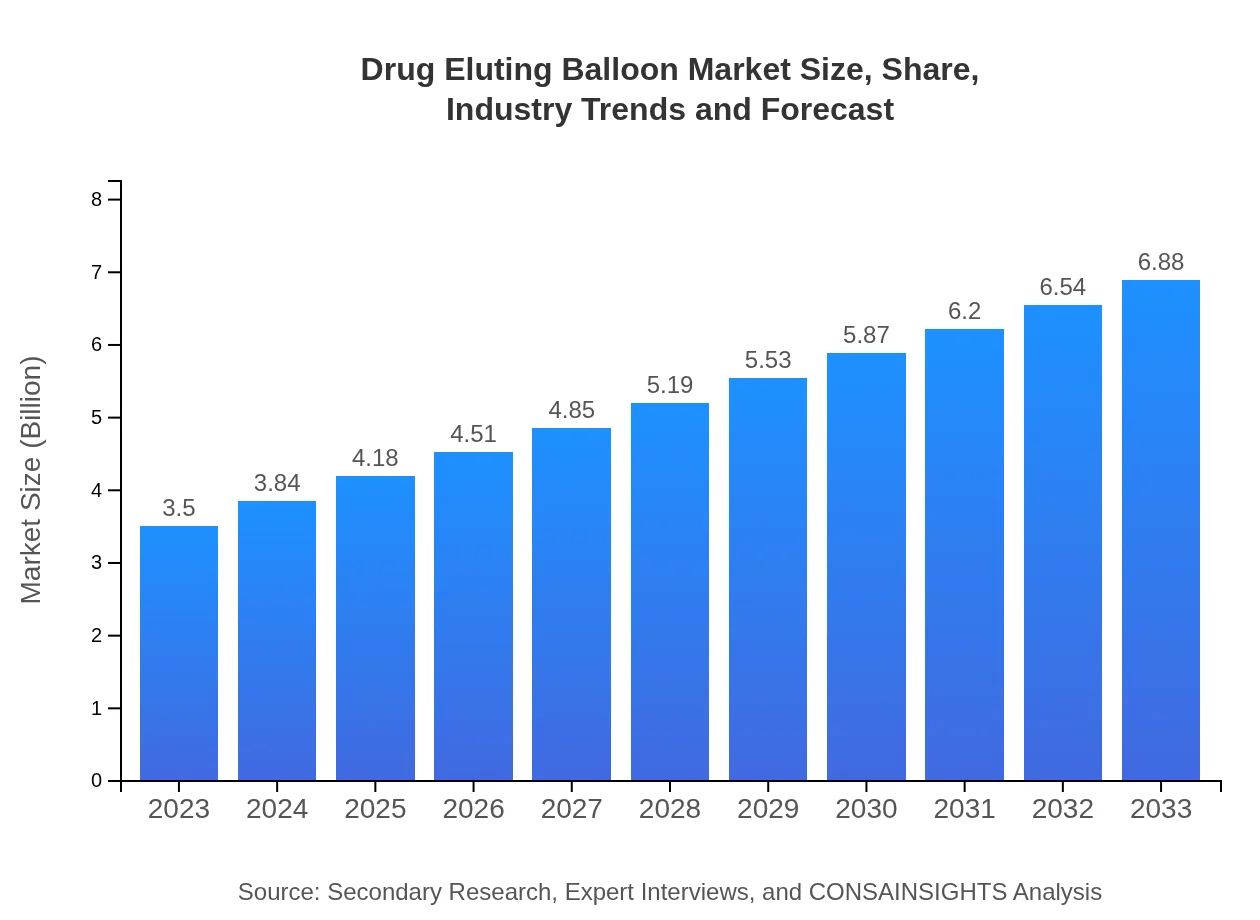
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Medtronic , Boston Scientific, C. R. Bard (part of BD), Abbott Laboratories, Cook Medical |
| Last Modified Date | 31 January 2026 |
Drug Eluting Balloon Market Overview
Customize Drug Eluting Balloon Market Report market research report
- ✔ Get in-depth analysis of Drug Eluting Balloon market size, growth, and forecasts.
- ✔ Understand Drug Eluting Balloon's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Drug Eluting Balloon
What is the Market Size & CAGR of Drug Eluting Balloon market in 2023?
Drug Eluting Balloon Industry Analysis
Drug Eluting Balloon Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Drug Eluting Balloon Market Analysis Report by Region
Europe Drug Eluting Balloon Market Report:
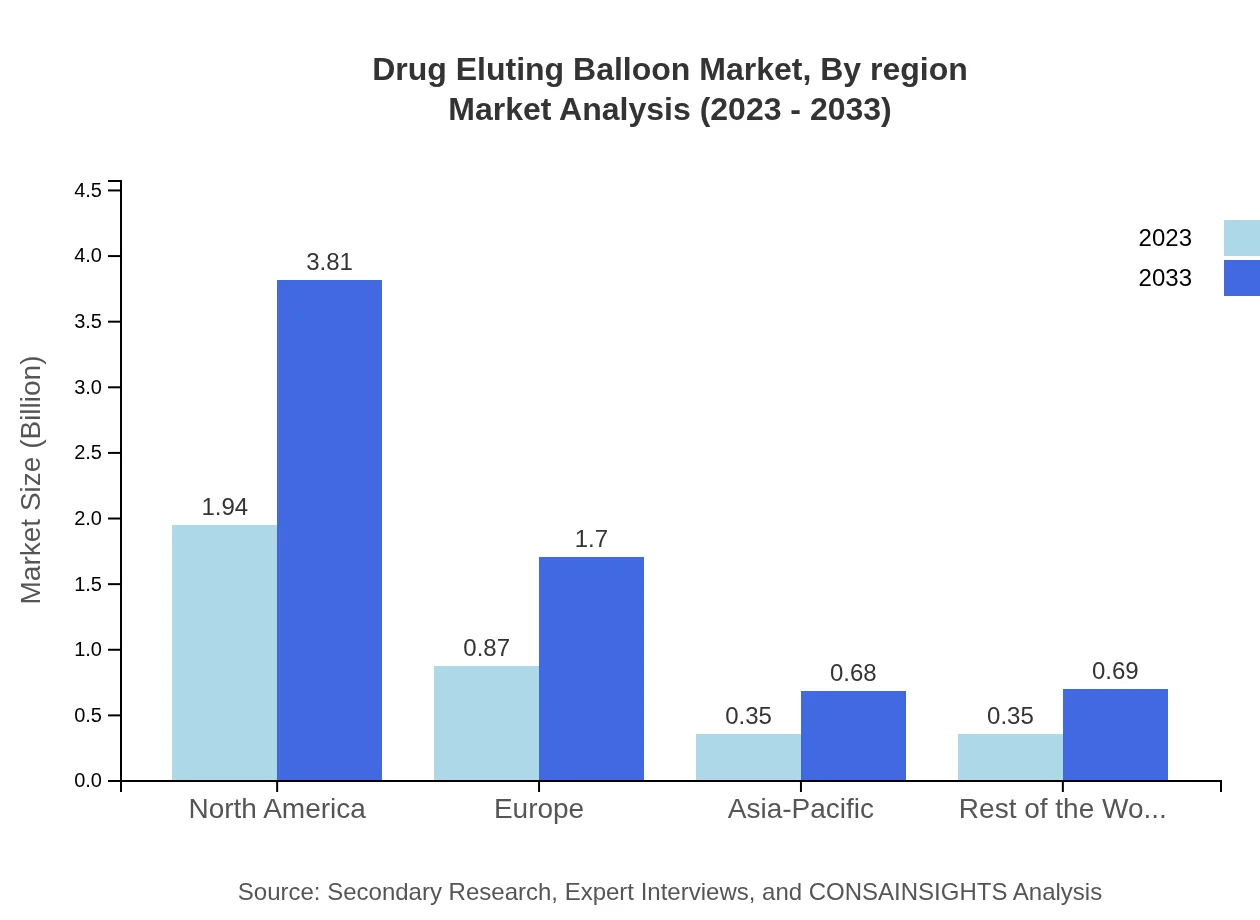
The European market is anticipated to grow from USD 1.01 billion in 2023 to USD 1.98 billion, primarily due to increasing awareness and demand for less invasive procedures, coupled with favorable reimbursement policies.Asia Pacific Drug Eluting Balloon Market Report:
The Asia-Pacific region is experiencing rapid growth, with a market size projected to rise from USD 0.69 billion in 2023 to USD 1.36 billion by 2033. This growth is fueled by increasing healthcare expenditures, a growing geriatric population, and rising incidences of cardiovascular diseases.North America Drug Eluting Balloon Market Report:
North America currently holds a significant share, with market size expecting to grow from USD 1.17 billion in 2023 to USD 2.30 billion by 2033. The growth is driven by a well-established healthcare system, increasing prevalence of coronary diseases, and the presence of key industry players.South America Drug Eluting Balloon Market Report:
In South America, the Drug Eluting Balloon market is projected to grow from USD 0.19 billion to USD 0.37 billion. The region is focusing on enhancing healthcare infrastructure, which will drive the adoption of advanced treatment options.Middle East & Africa Drug Eluting Balloon Market Report:
The Middle East and Africa region expect growth as well, with a projected increase from USD 0.44 billion to USD 0.86 billion by 2033, facilitated by rising healthcare investments and improving access to advanced medical technologies.Tell us your focus area and get a customized research report.
Drug Eluting Balloon Market Analysis By Device Type
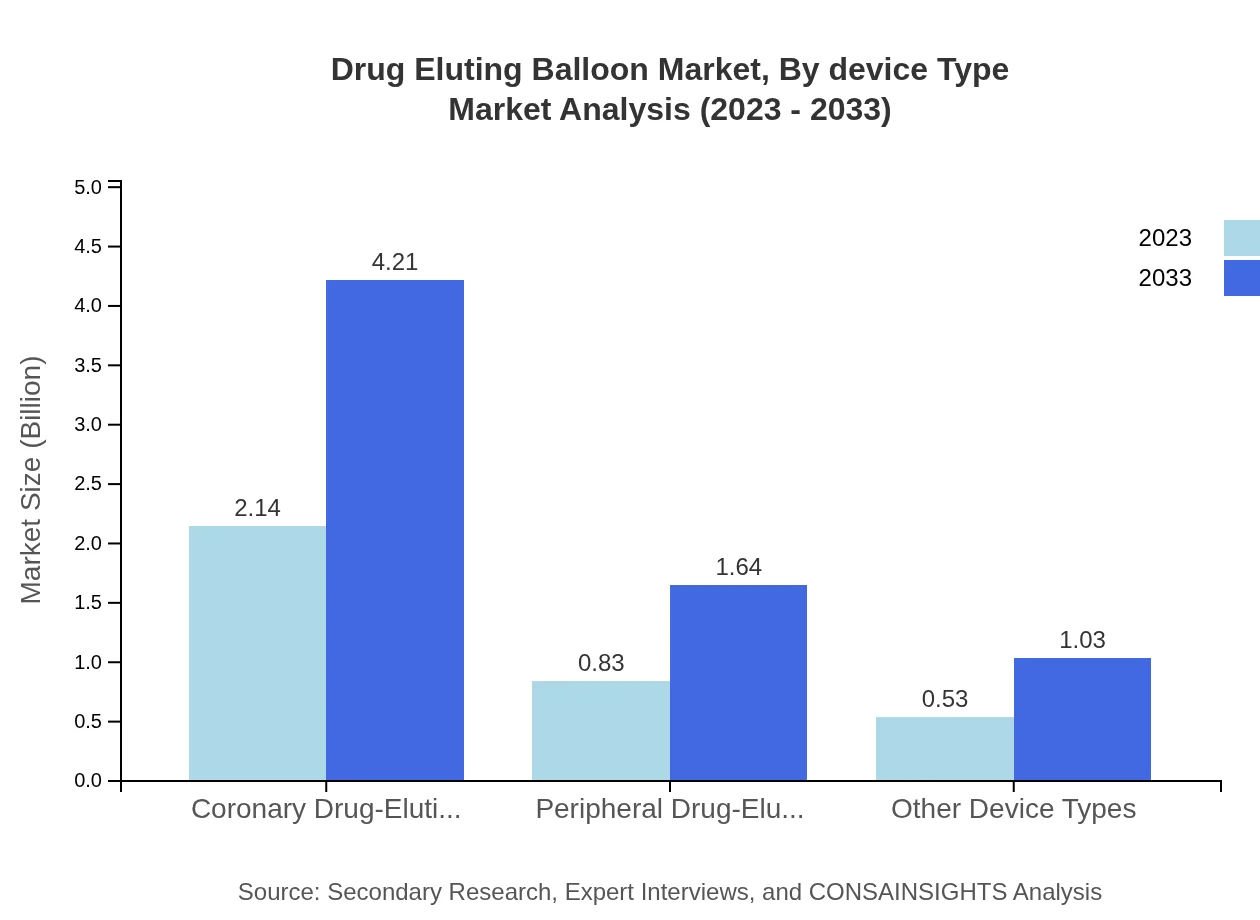
In 2023, the market for Drug Eluting Balloons segmented by device type shows a strong inclination towards coronary drug-eluting balloons, expected to grow from USD 2.14 billion to USD 4.21 billion by 2033, capturing 61.15% of the market share consistently over the years. Peripheral drug-eluting balloons hold a significant market position as well, with forecasts indicating growth from USD 0.83 billion to USD 1.64 billion, making up 23.85% of the market.
Drug Eluting Balloon Market Analysis By Application
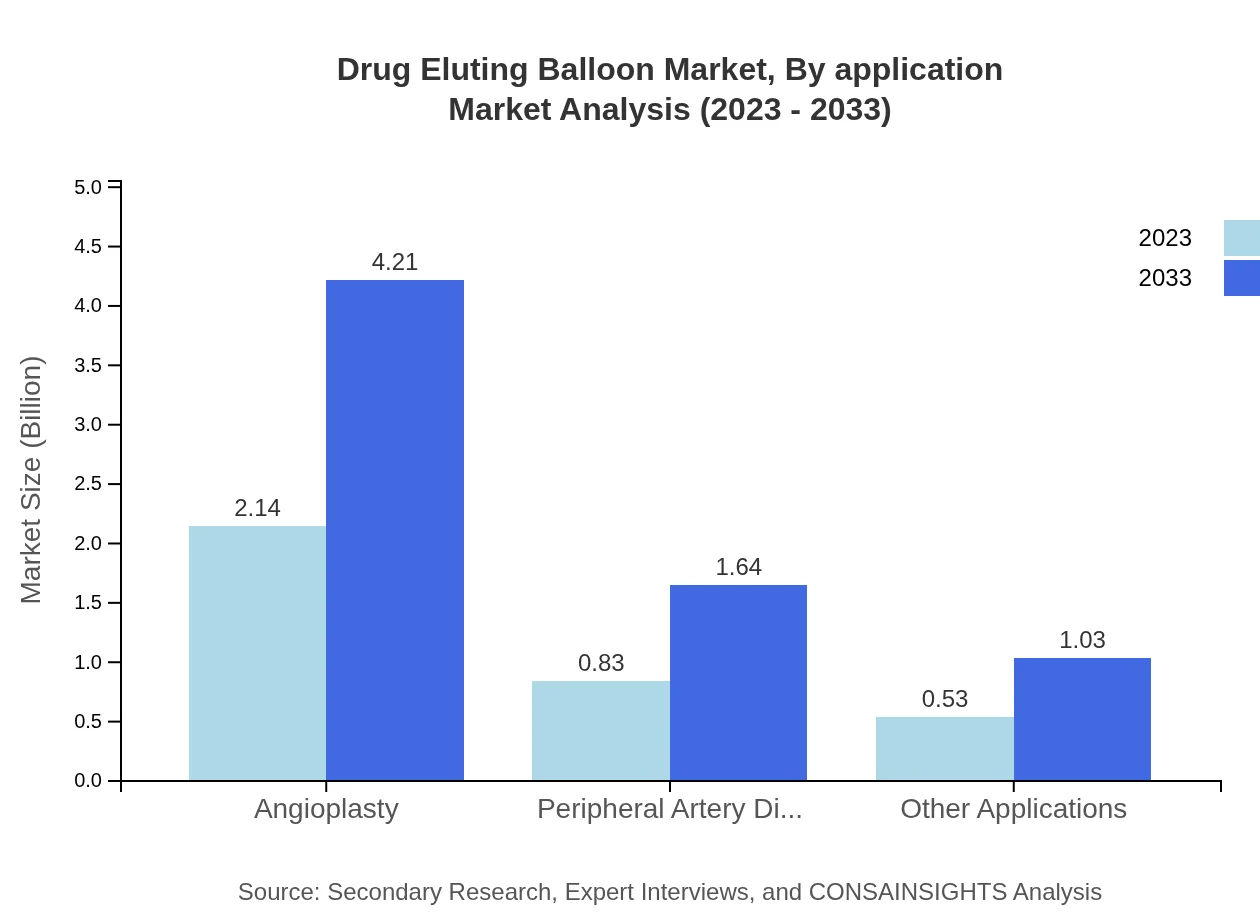
The application-based segmentation reveals that coronary artery disease treatment remains the dominant segment, projected to account for a majority market share. Peripheral artery disease applications are also crucial, anticipated to grow significantly in both size and share, underlining the importance of diverse medical needs met by drug-eluting balloons.
Drug Eluting Balloon Market Analysis By End User
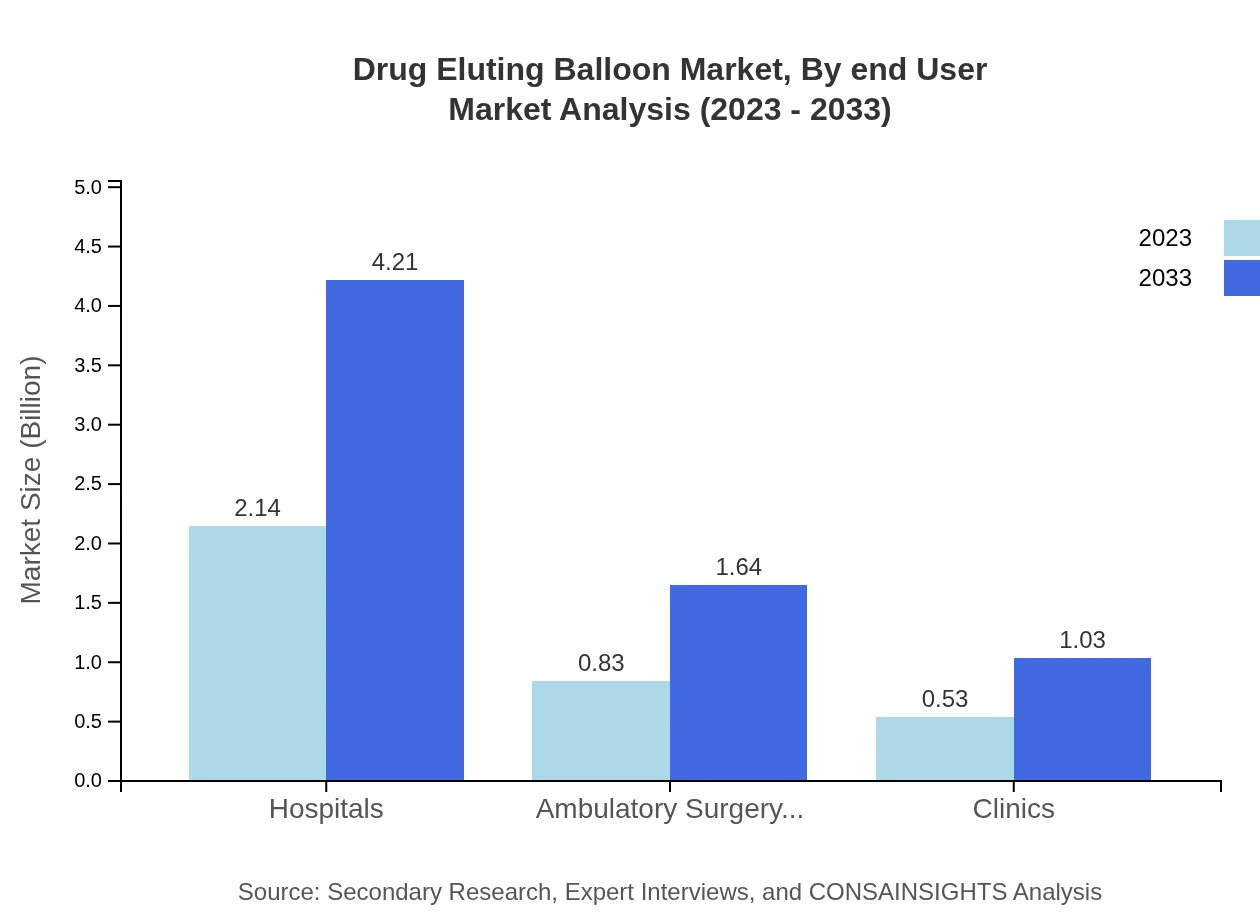
Hospitals remain the largest end-user segment, with market size expected to rise from USD 2.14 billion to USD 4.21 billion. They contribute to 61.15% of the market share, followed closely by ambulatory surgery centers and clinics, each catering to the scaling demand for outpatient and minimally invasive procedures.
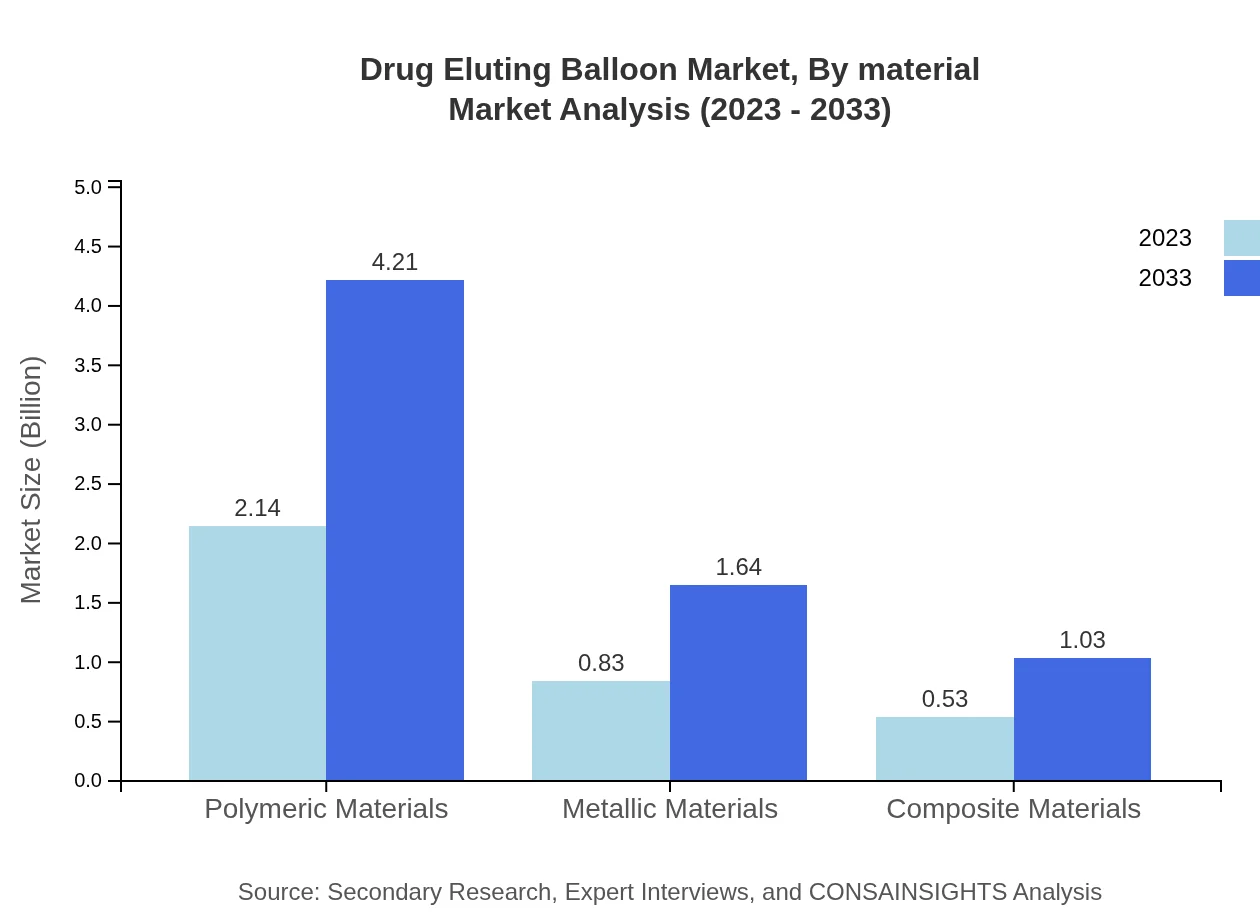
Drug Eluting Balloon Market Analysis By Material
The segmentation by material indicates a significant market for polymeric materials, which are expected to dominate in both size and share. Currently, they represent 61.15% of the market, while metallic and composite materials possess a smaller, yet relevant, share, highlighting innovations in material science affecting product performance and patient outcomes.
Drug Eluting Balloon Market Analysis By Region
Regionally, North America holds the largest market share while Europe and Asia-Pacific are rapidly growing segments. The rest of the world, particularly South America and the Middle East, are emerging markets showcasing substantial growth opportunities due to increasing healthcare investments and improved distribution channels.
Drug Eluting Balloon Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Drug Eluting Balloon Industry
Medtronic :
Medtronic is a leading player in the medical devices sector, offering innovative drug-eluting balloons aimed at treating coronary artery disease with proven efficacy and safety profiles.Boston Scientific:
Boston Scientific is renowned for its cutting-edge medical solutions, focusing extensively on developing advanced drug-eluting balloon technologies to improve patient outcomes in cardiovascular therapy.C. R. Bard (part of BD):
With a long legacy in vascular intervention, C. R. Bard integrates drug-eluting technology into their balloon intervention products, addressing critical needs in peripheral artery disease management.Abbott Laboratories:
Abbott maintains a significant presence in the drug-eluting balloon market through its innovative products that enhance the treatment landscape of cardiovascular diseases.Cook Medical:
Cook Medical focuses on providing innovative solutions in the vascular sector, including drug-eluting balloons that cater to complex interventional needs.We're grateful to work with incredible clients.









FAQs
What is the market size of drug Eluting Balloon?
The global drug-eluting balloon market is currently valued at approximately $3.5 billion and is projected to grow at a CAGR of 6.8% over the next decade, indicating robust demand and expansion in this sector.
What are the key market players or companies in this drug Eluting Balloon industry?
Key players in the drug-eluting balloon market include large medical device manufacturers such as Abbott Laboratories, Medtronic, and Boston Scientific, which are recognized for their innovative products and significant market shares.
What are the primary factors driving the growth in the drug Eluting Balloon industry?
The growth of the drug-eluting balloon market is primarily driven by increasing prevalence of cardiovascular diseases, innovation in medical technology, and rising geriatric populations requiring advanced therapeutic solutions.
Which region is the fastest Growing in the drug Eluting Balloon?
The Asia Pacific region is among the fastest-growing markets for drug-eluting balloons, with its size expected to grow from $0.69 billion in 2023 to $1.36 billion by 2033, reflecting a surge in healthcare advancements.
Does ConsaInsights provide customized market report data for the drug Eluting Balloon industry?
Yes, ConsaInsights offers customized market report data tailored to client-specific inquiries within the drug-eluting balloon industry, ensuring that stakeholders receive the most relevant and actionable insights.
What deliverables can I expect from this drug Eluting Balloon market research project?
Deliverables from the drug-eluting balloon market research project include comprehensive reports detailing market size, segmentation, growth trends, and competitive analysis to guide strategic decision-making for stakeholders.
What are the market trends of drug Eluting Balloon?
Current trends in the drug-eluting balloon market include a shift towards new materials and technologies, increasing investment in R&D, and a growing focus on minimally invasive procedures to enhance patient outcomes.