Drug Eluting Stent Market Report
Published Date: 31 January 2026 | Report Code: drug-eluting-stent
Drug Eluting Stent Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Drug Eluting Stent market from 2023 to 2033, covering market trends, regional insights, segmentation, and forecasts, along with a focused exploration of industry leaders and technological advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
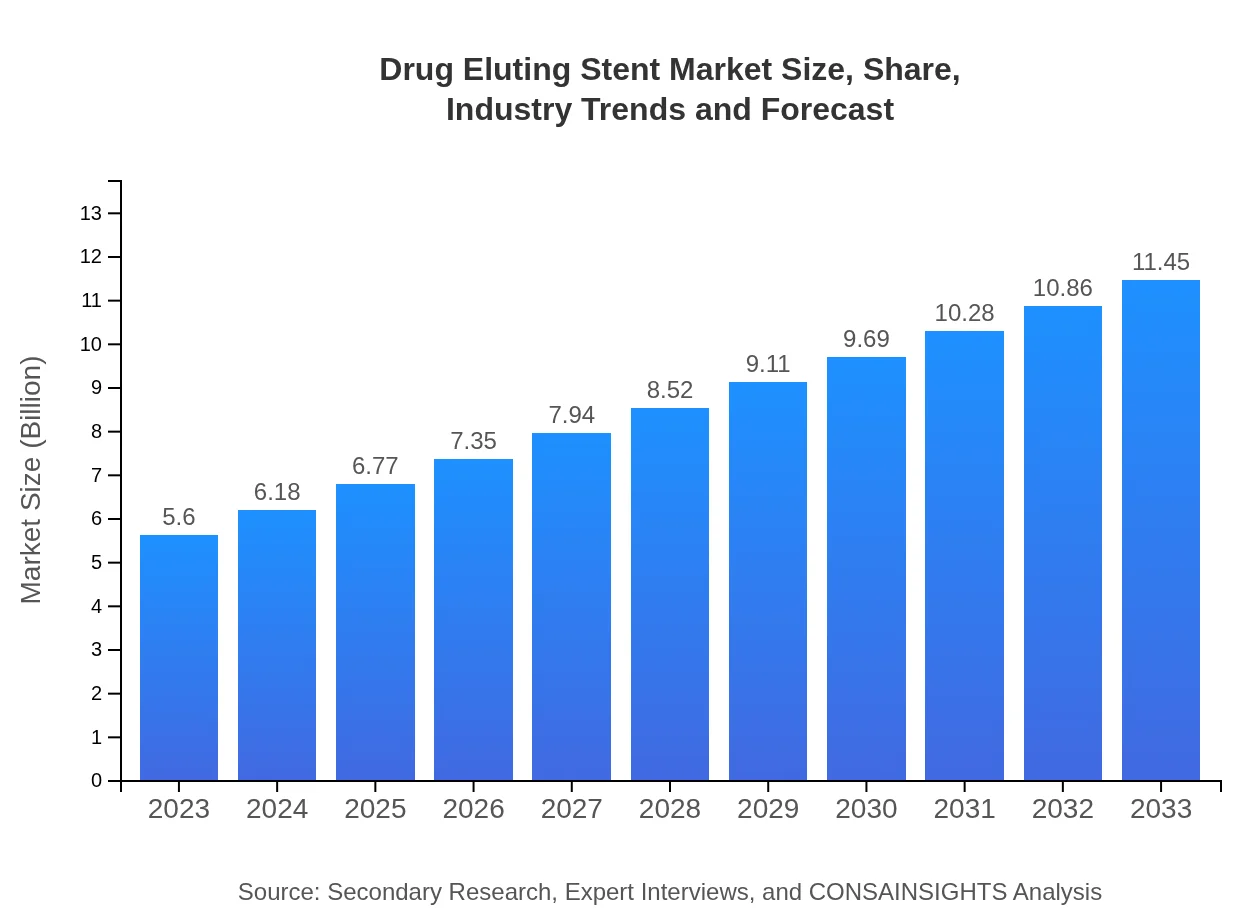
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | Abbott Laboratories, Boston Scientific Corporation, Medtronic , Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Drug Eluting Stent Market Overview
Customize Drug Eluting Stent Market Report market research report
- ✔ Get in-depth analysis of Drug Eluting Stent market size, growth, and forecasts.
- ✔ Understand Drug Eluting Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Drug Eluting Stent
What is the Market Size & CAGR of Drug Eluting Stent market in 2023?
Drug Eluting Stent Industry Analysis
Drug Eluting Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Drug Eluting Stent Market Analysis Report by Region
Europe Drug Eluting Stent Market Report:
The European region shows promising growth, beginning at USD 1.68 billion in 2023 and projected to reach USD 3.44 billion by 2033. High healthcare spending, a growing elderly population, and increasing patient demand for effective cardiovascular treatments are key growth drivers.Asia Pacific Drug Eluting Stent Market Report:
The Asia Pacific region is experiencing substantial growth in the Drug Eluting Stent market, projected to grow from USD 1.12 billion in 2023 to USD 2.29 billion by 2033. Factors driving this growth include rising healthcare expenditures, increased adoption of advanced healthcare technologies, and a higher incidence of cardiovascular diseases.North America Drug Eluting Stent Market Report:
North America continues to dominate the Drug Eluting Stent market, with an estimated market size of USD 1.90 billion in 2023 expected to reach USD 3.87 billion by 2033. The region's leadership is attributed to advanced healthcare technologies, significant investments in R&D, and a higher prevalence of cardiovascular disease.South America Drug Eluting Stent Market Report:
The South American market for Drug Eluting Stents is expected to develop modestly, rising from USD 0.29 billion in 2023 to USD 0.60 billion by 2033. Factors such as improving healthcare infrastructure and rising awareness of cardiovascular treatments contribute to this market expansion.Middle East & Africa Drug Eluting Stent Market Report:
The Middle East and Africa market is expected to gradually improve, from USD 0.61 billion in 2023 to USD 1.25 billion by 2033. Improved health policies, better access to innovative products, and increased healthcare spending drive this region’s growth.Tell us your focus area and get a customized research report.
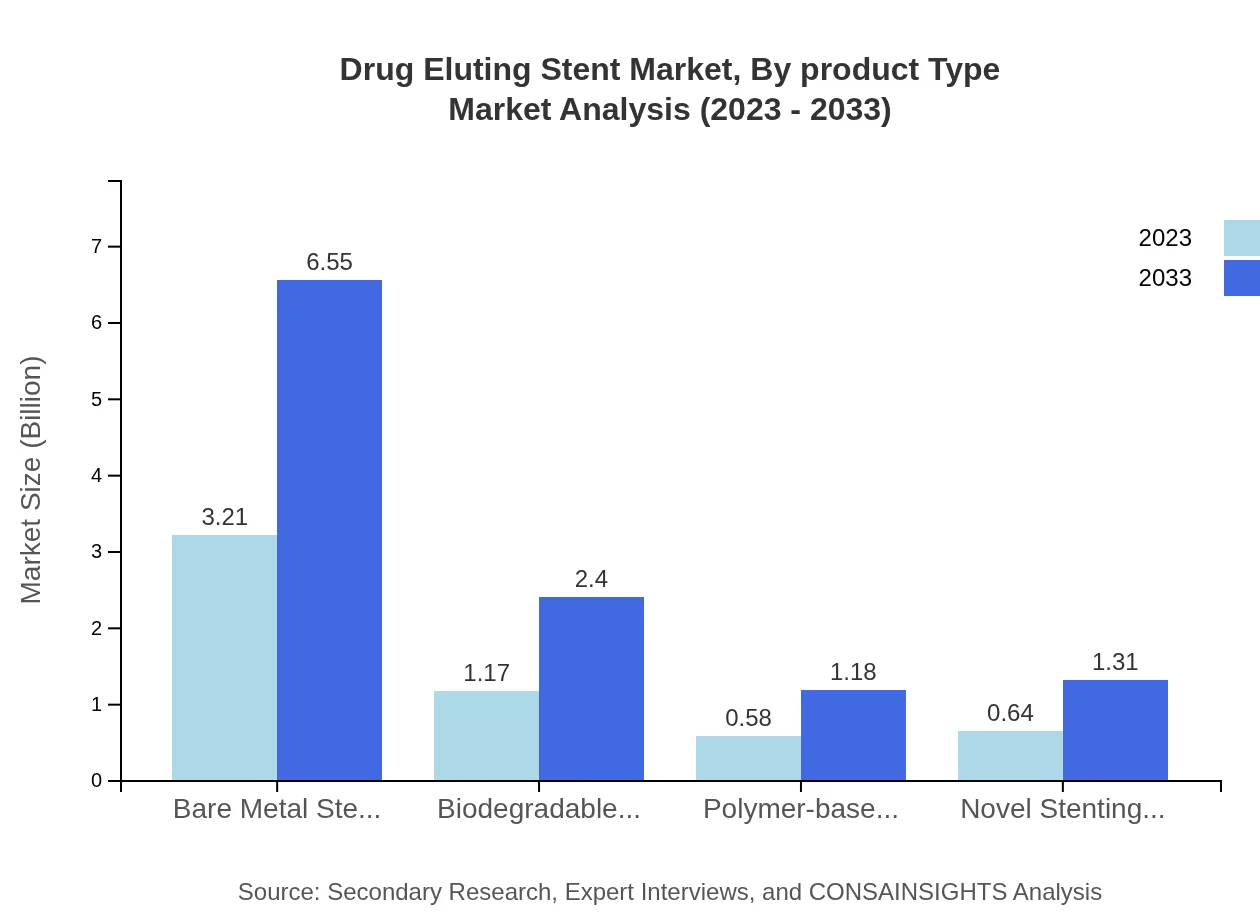
Drug Eluting Stent Market Analysis By Product Type
The product type segment shows notable growth, with Everolimus stents dominating the market. By 2033, the Everolimus stent market is projected to grow from USD 3.62 billion in 2023 to USD 7.39 billion, retaining a market share of 64.56%. Zotarolimus stents also show growth from USD 1.33 billion to USD 2.72 billion capturing 23.76% of the market by 2033. Other types like biodegradable and polymer-based stents are gradually gaining traction as healthcare shifts towards patient-centric solutions.
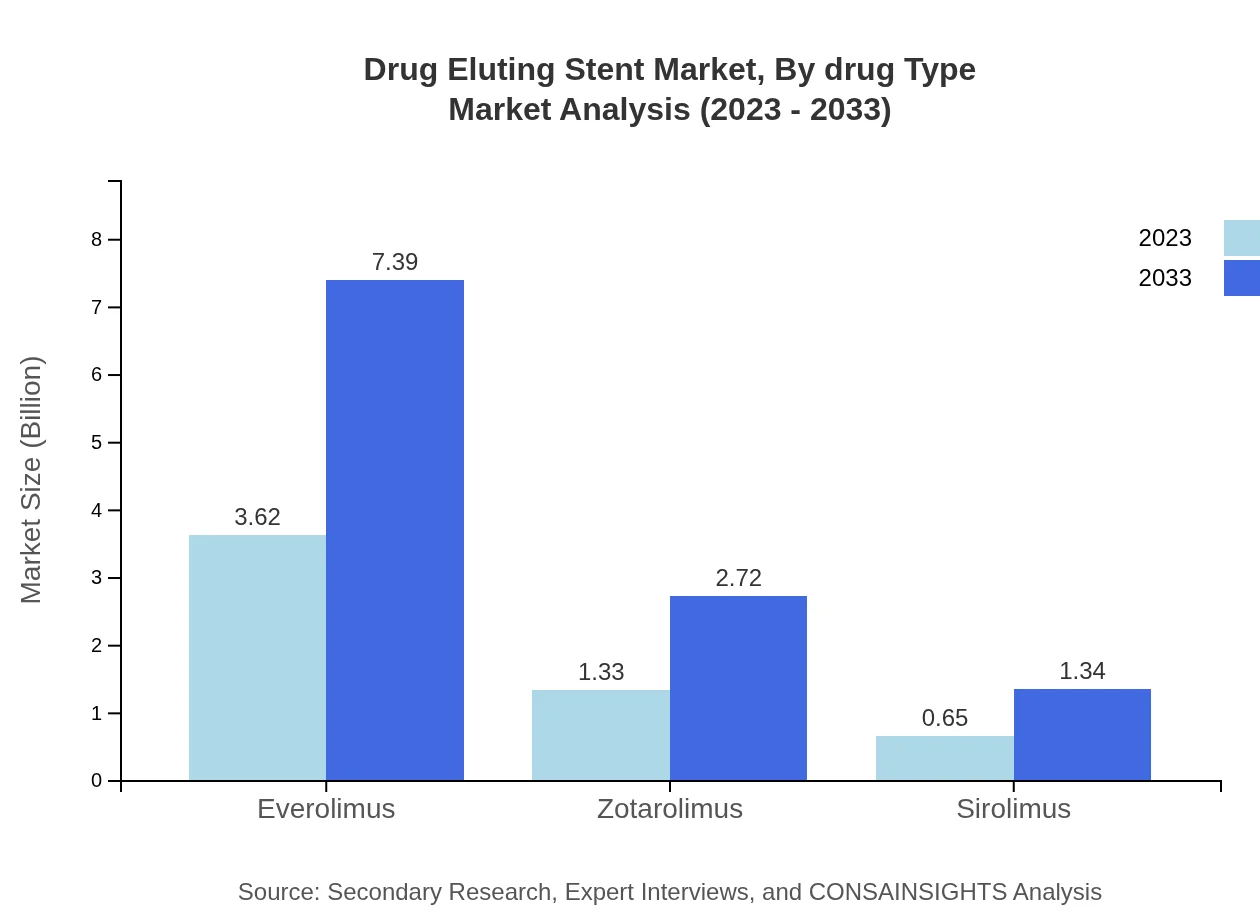
Drug Eluting Stent Market Analysis By Drug Type
In the drug type segment, the Everolimus drug type leads the market, with projections showing an increase from USD 3.62 billion in 2023 to USD 7.39 billion by 2033. The market share remains steady at 64.56%. Zotarolimus also shows incremental growth, from USD 1.33 billion to USD 2.72 billion, maintaining 23.76% market share. The use of Sirolimus and biodegradable drugs is also expected to change over the forecast period, reflecting growing research and development initiatives.
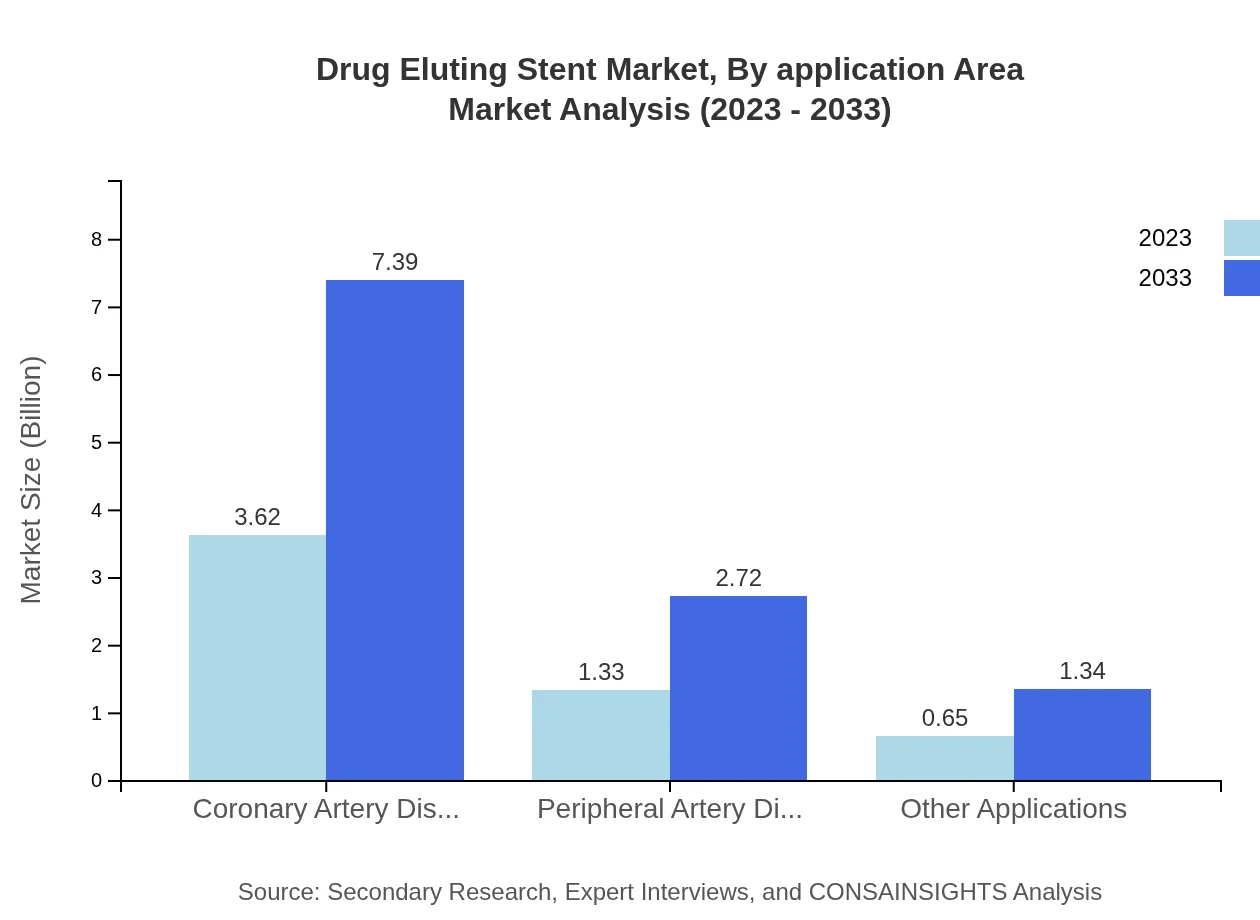
Drug Eluting Stent Market Analysis By Application Area
The application area segment is robust, with coronary artery disease being the primary application, growing from USD 3.62 billion in 2023 to USD 7.39 billion by 2033. This segment retains its share of 64.56%. The peripheral artery disease sector is also noteworthy, growing from USD 1.33 billion to USD 2.72 billion by 2033, with steady interest in other applications, showcasing the versatility of Drug Eluting Stents.
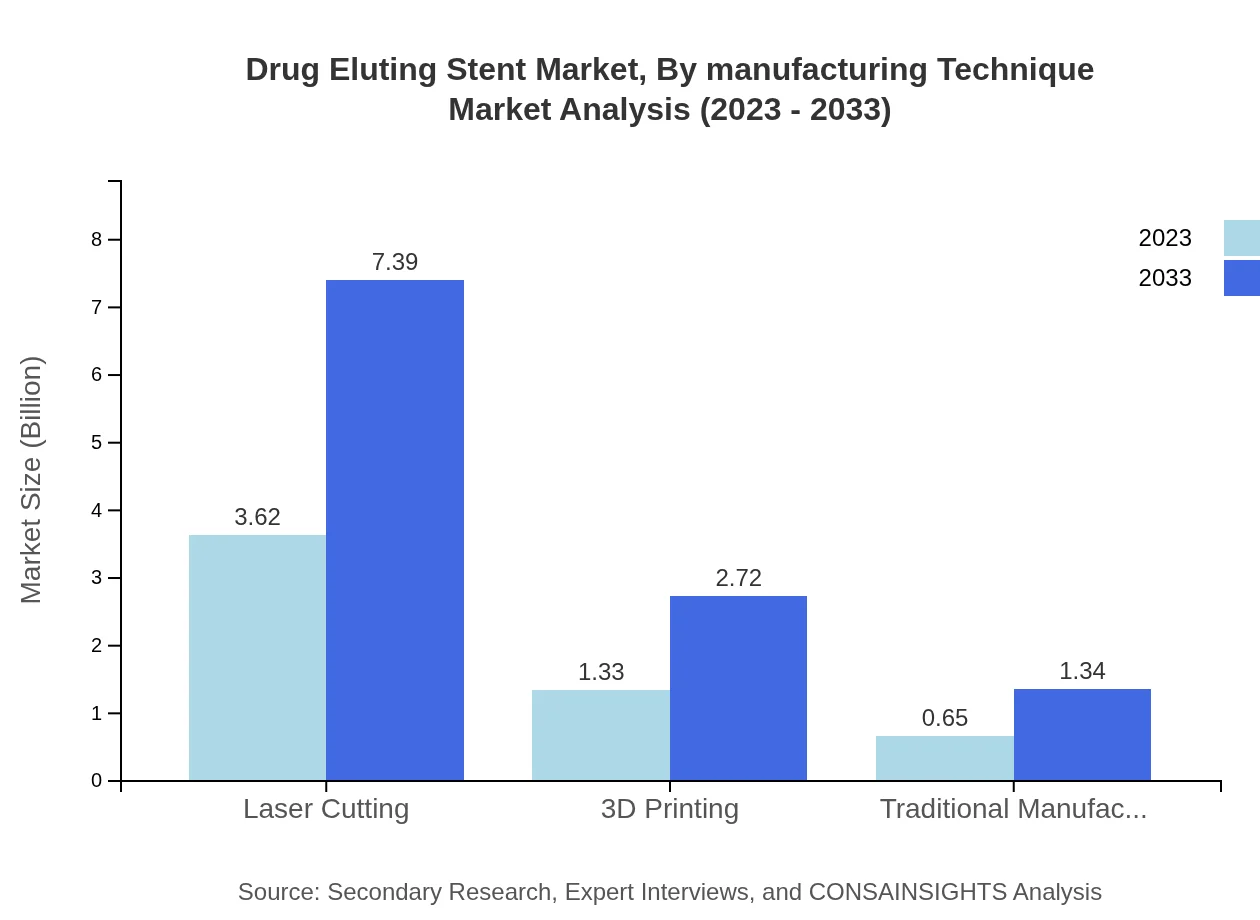
Drug Eluting Stent Market Analysis By Manufacturing Technique
Among manufacturing techniques, traditional manufacturing methods continue to hold a significant market share, while 3D printing technologies are emerging as innovative alternatives. The 3D printing market is expected to expand from USD 1.33 billion in 2023 to USD 2.72 billion by 2033, capturing 23.76% of the segment. Laser cutting also holds considerable market weight, maintaining its share throughout the forecast period, although innovation-focused companies are pivoting towards modern manufacturing techniques to improve efficiency and efficacy.
Drug Eluting Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Drug Eluting Stent Industry
Abbott Laboratories:
Abbott is a global healthcare leader, known for its cutting-edge medical technologies, including Drug Eluting Stents. The company's innovative product lines enhance patient outcomes and offer impressive efficacy and safety.Boston Scientific Corporation:
Boston Scientific is a key player in the cardiovascular space, particularly in Drug Eluting Stents, and focuses on developing advanced therapies that meet the needs of patients and healthcare providers.Medtronic :
Medtronic is recognized for its innovative cardiovascular devices, pioneering stent technology that has advanced treatment options for coronary artery disease and improved patient care globally.Siemens Healthineers:
A leader in diagnostic imaging and advanced therapies, Siemens Healthineers provides innovative solutions and products that contribute to improved procedural outcomes with DES technology.We're grateful to work with incredible clients.









FAQs
What is the market size of drug Eluting stents?
The drug-eluting stent market is valued at approximately $5.6 billion in 2023 and is projected to grow at a CAGR of 7.2%, reaching significant market size by 2033.
What are the key market players or companies in the drug Eluting stent industry?
Key players in the drug-eluting stent industry include Medtronic, Abbott Laboratories, Boston Scientific, and Cordis Corporation, among others, leading the innovation and development of advanced stenting technologies.
What are the primary factors driving the growth in the drug Eluting stent industry?
Growth drivers for the drug-eluting stent industry include rising prevalence of coronary artery disease, technological advancements, increasing healthcare expenditure, and greater adoption of minimally invasive procedures.
Which region is the fastest Growing in the drug Eluting stent market?
Asia-Pacific is the fastest-growing region for drug-eluting stents, with a market size forecasted to grow from $1.12 billion in 2023 to $2.29 billion by 2033, reflecting increasing healthcare access and demand.
Does ConsaInsights provide customized market report data for the drug Eluting stent industry?
Yes, ConsaInsights offers customized market reports tailored to specific queries and needs, providing insightful data and analytics for stakeholders in the drug-eluting stent market.
What deliverables can I expect from this drug Eluting stent market research project?
Expected deliverables from the drug-eluting stent market research project include detailed market analysis, growth forecasts, competitive landscape assessments, and comprehensive segment breakdowns.
What are the market trends of drug Eluting stents?
Current trends in the drug-eluting stent market include the rise of biodegradable stents, increasing focus on drug-coated technologies, and advancements in manufacturing techniques like 3D printing.