Dual Chamber Prefilled Syringes Market Report
Published Date: 31 January 2026 | Report Code: dual-chamber-prefilled-syringes
Dual Chamber Prefilled Syringes Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Dual Chamber Prefilled Syringes market from 2023 to 2033. It covers market size, growth trends, regional insights, industry analysis, and competitive landscape, equipping stakeholders with critical data for informed decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
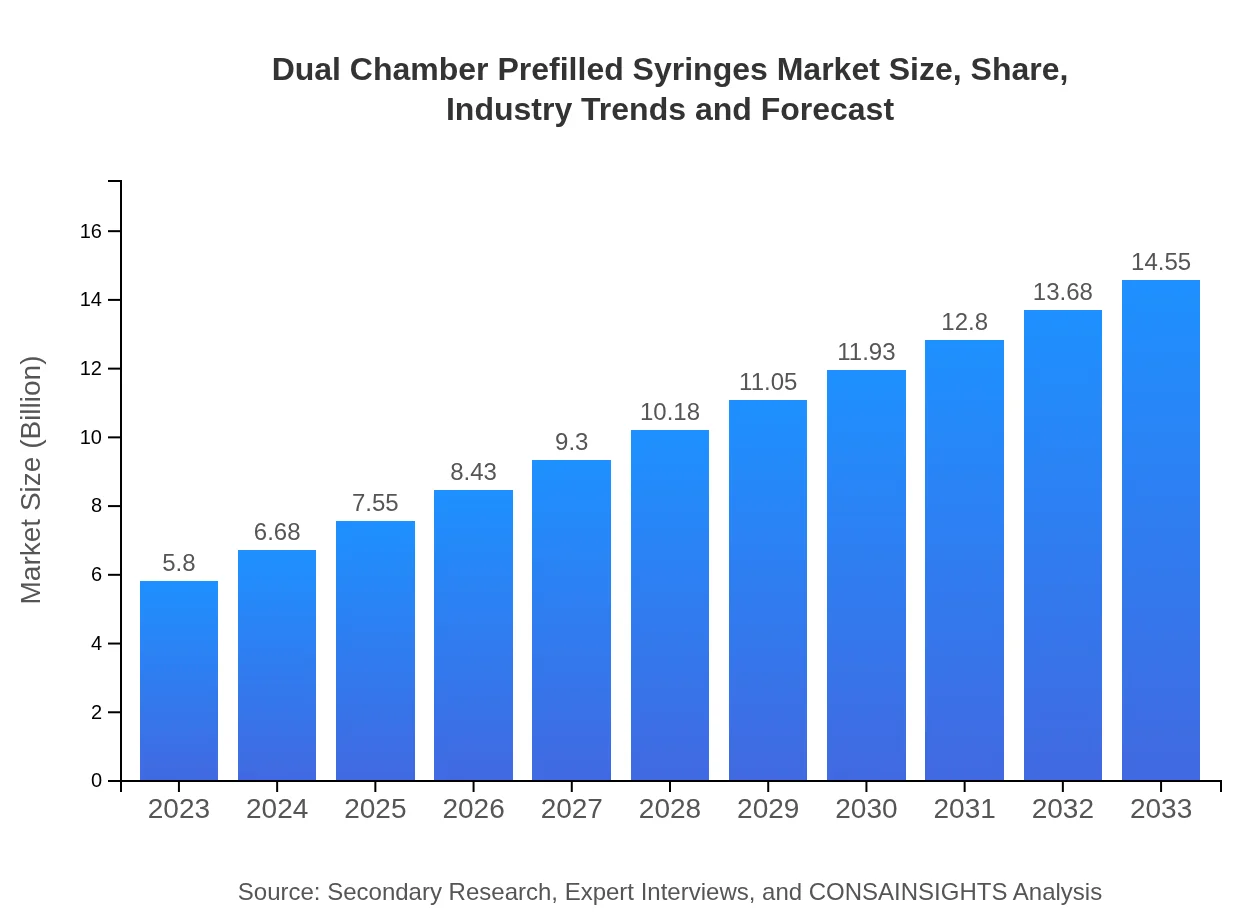
| 2023 Market Size | $5.80 Billion |
| CAGR (2023-2033) | 9.3% |
| 2033 Market Size | $14.55 Billion |
| Top Companies | BD (Becton, Dickinson and Company), Gerresheimer AG, Schott AG, West Pharmaceutical Services, Inc. |
| Last Modified Date | 31 January 2026 |
Dual Chamber Prefilled Syringes Market Overview
Customize Dual Chamber Prefilled Syringes Market Report market research report
- ✔ Get in-depth analysis of Dual Chamber Prefilled Syringes market size, growth, and forecasts.
- ✔ Understand Dual Chamber Prefilled Syringes's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Dual Chamber Prefilled Syringes
What is the Market Size & CAGR of Dual Chamber Prefilled Syringes market in 2023?
Dual Chamber Prefilled Syringes Industry Analysis
Dual Chamber Prefilled Syringes Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Dual Chamber Prefilled Syringes Market Analysis Report by Region
Europe Dual Chamber Prefilled Syringes Market Report:
The European market is expected to rise from $1.49 billion in 2023 to $3.73 billion by 2033. The region benefits from rigorous regulatory frameworks and a focus on enhancing patient safety and treatment outcomes.Asia Pacific Dual Chamber Prefilled Syringes Market Report:
In the Asia Pacific region, the Dual Chamber Prefilled Syringes market is expected to grow from $1.22 billion in 2023 to $3.05 billion by 2033. The growth can be attributed to increasing healthcare expenditures, rising awareness regarding advanced drug delivery mechanisms, and expanding biopharmaceutical research activities.North America Dual Chamber Prefilled Syringes Market Report:
In North America, the market will grow significantly from $2.12 billion in 2023 to $5.32 billion in 2033. This growth is driven by a high prevalence of chronic diseases, the presence of major pharmaceutical companies, and advancements in drug delivery technology.South America Dual Chamber Prefilled Syringes Market Report:
The South American market for Dual Chamber Prefilled Syringes is projected to increase from $0.42 billion in 2023 to $1.06 billion by 2033. Factors contributing to this growth include improving healthcare infrastructure and rising incidences of chronic diseases that require innovative treatment methods.Middle East & Africa Dual Chamber Prefilled Syringes Market Report:
The Middle East and Africa market will expand from $0.56 billion in 2023 to $1.39 billion by 2033. This expansion is fueled by growing investments in healthcare infrastructure and rising awareness about the benefits of prefilled syringes.Tell us your focus area and get a customized research report.
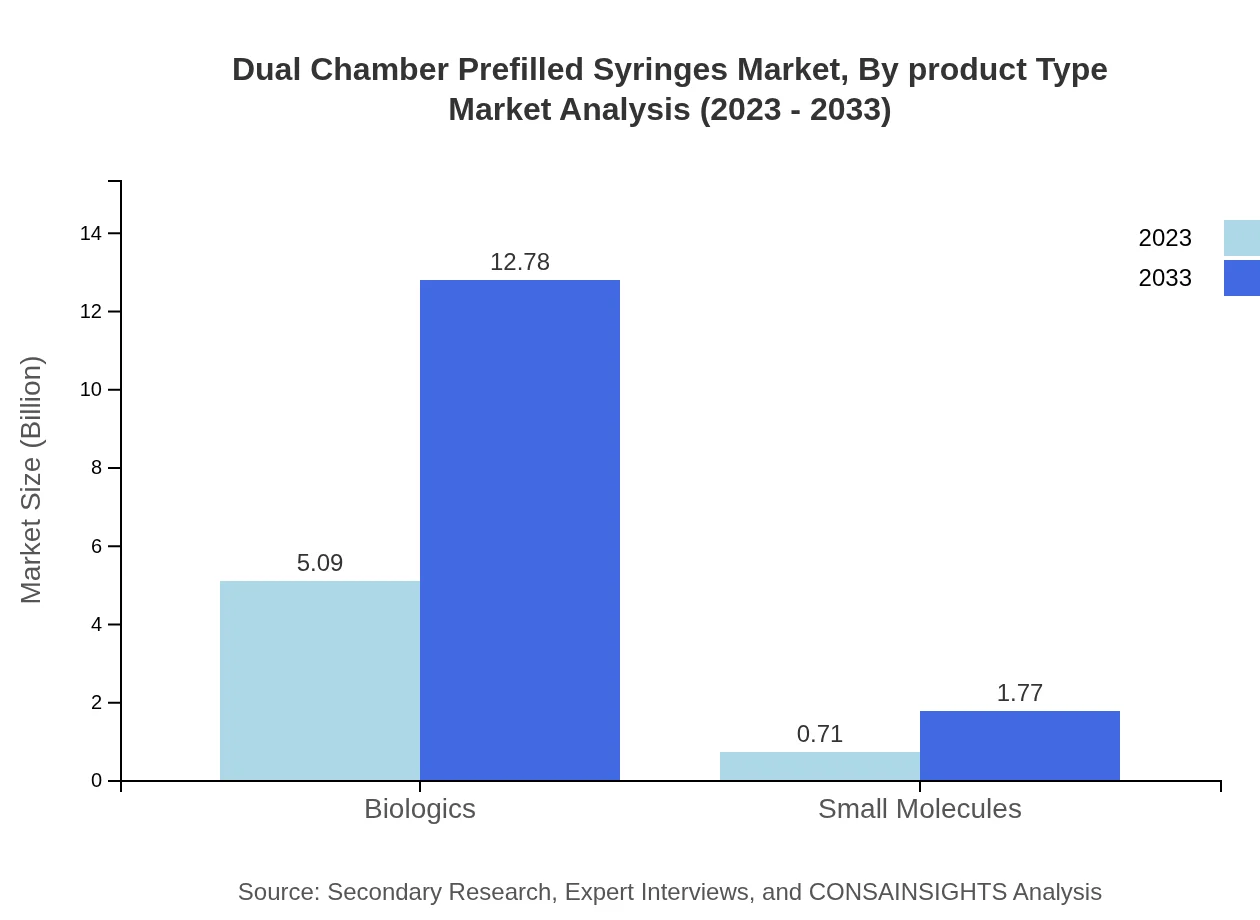
Dual Chamber Prefilled Syringes Market Analysis By Product Type
The Dual Chamber Prefilled Syringes market by product type includes biologics and small molecules. Biologics dominate the market due to their specific therapeutic applications, providing high efficacy in treatment for various serious health conditions. As a result, this segment offers significant growth potential throughout the forecast period.
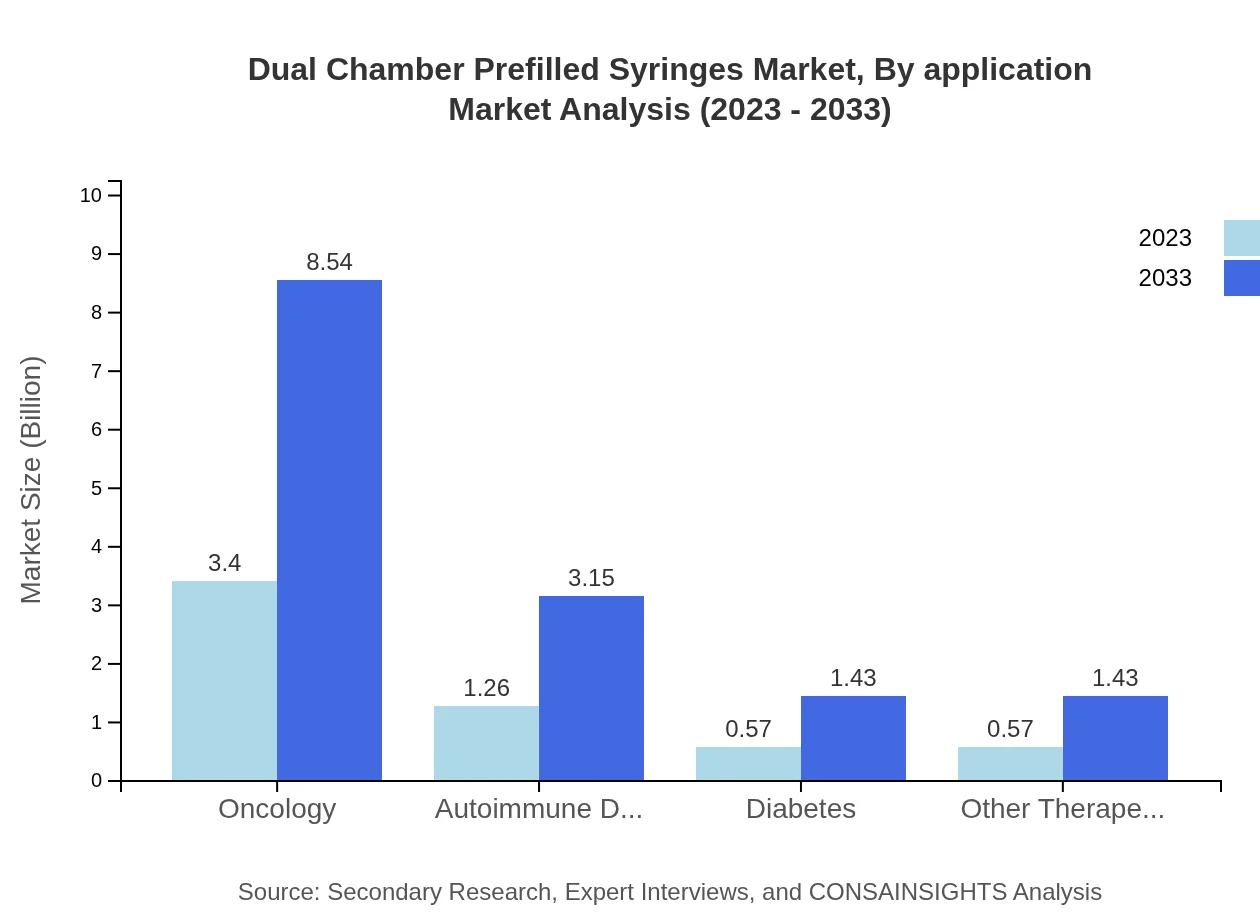
Dual Chamber Prefilled Syringes Market Analysis By Application
The applications of Dual Chamber Prefilled Syringes are diverse, with oncology representing the largest segment. Other applications, including autoimmune disorders and diabetes management, are also notable. The growing prevalence of these conditions drives demand for advanced delivery systems, highlighting the importance of innovation in this space.
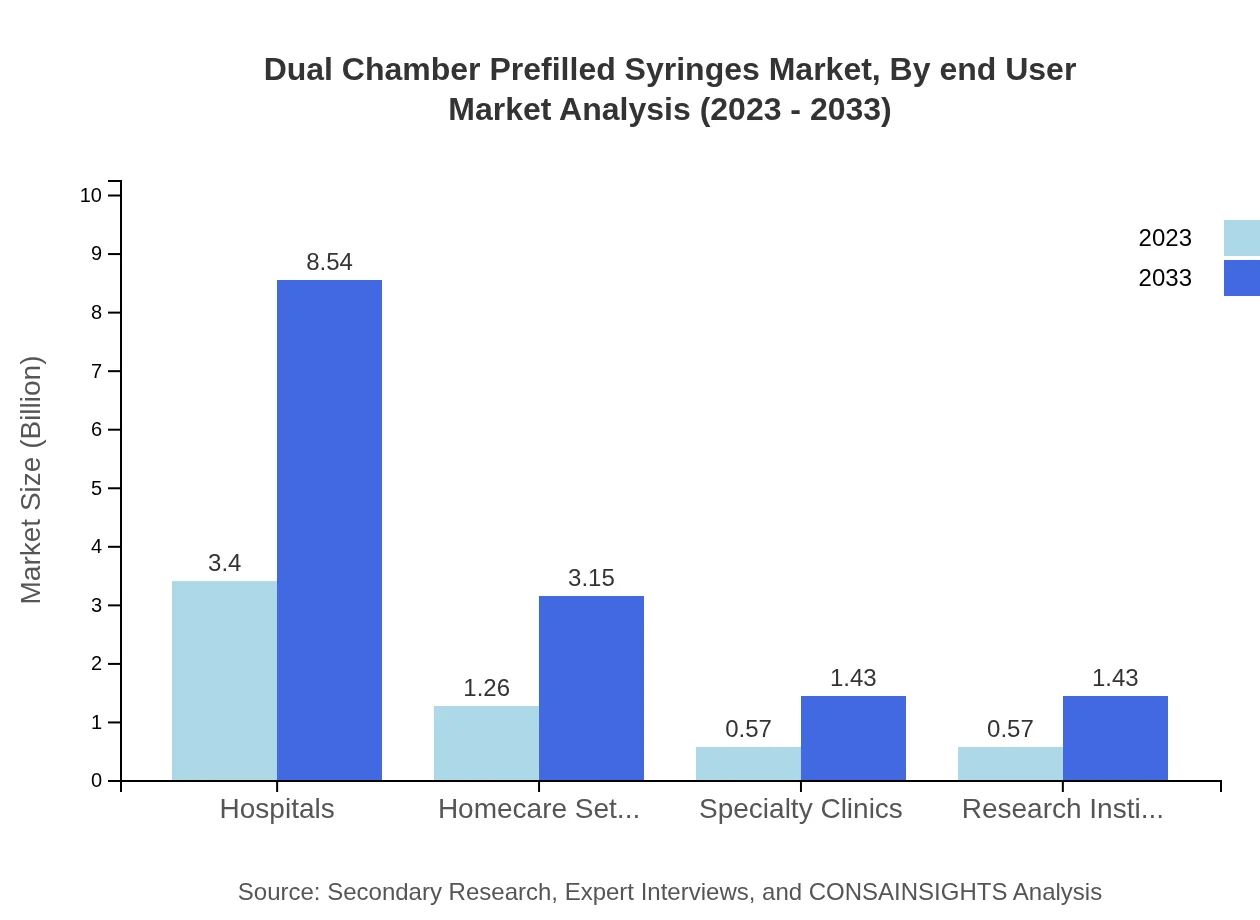
Dual Chamber Prefilled Syringes Market Analysis By End User
Hospitals remain the largest end-user segment of the Dual Chamber Prefilled Syringes market, accounting for a major share of the market. Homecare settings are also increasingly significant due to the rising preference for at-home treatment for chronic conditions. Specialty clinics and research institutions complement the market by providing focused therapeutic applications.
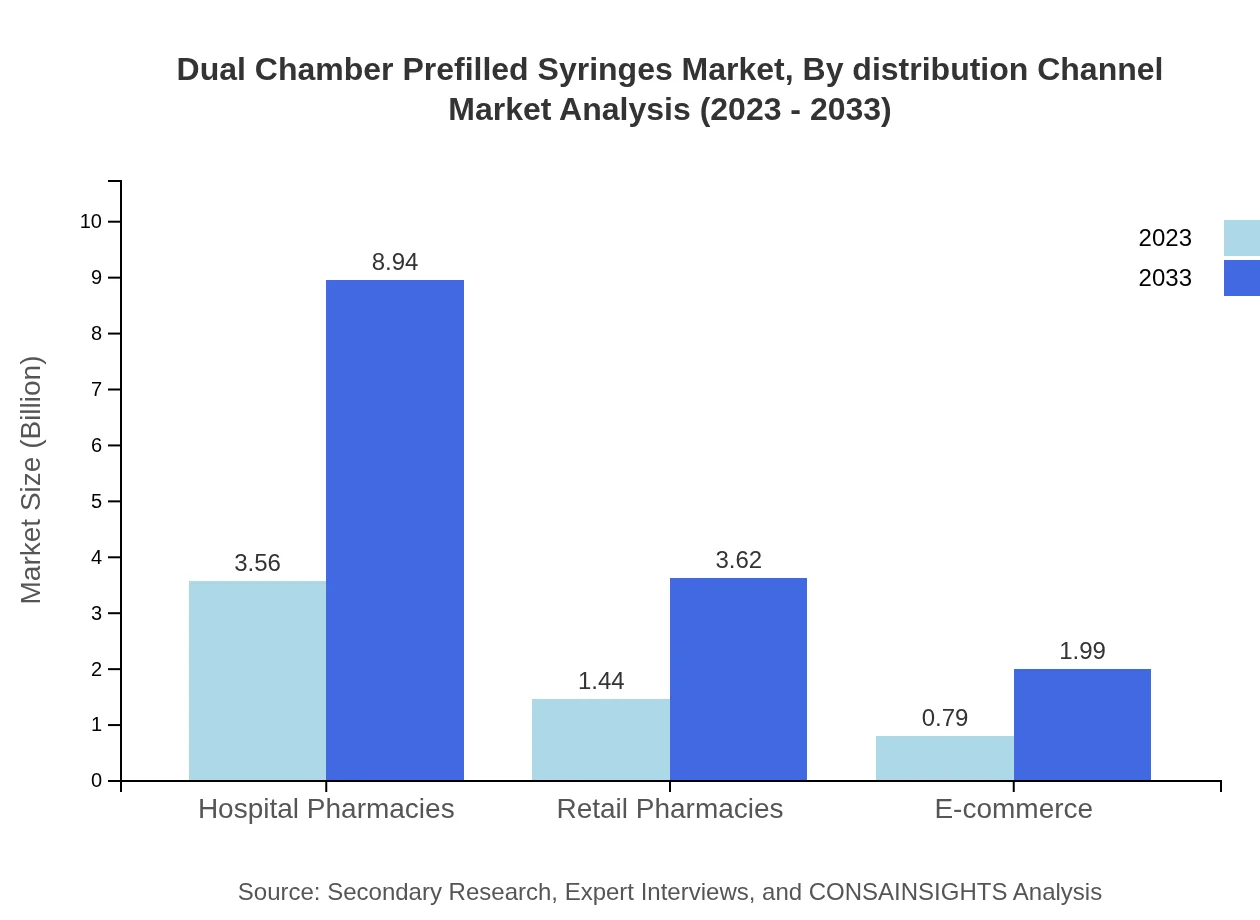
Dual Chamber Prefilled Syringes Market Analysis By Distribution Channel
Distribution channels for Dual Chamber Prefilled Syringes include hospital pharmacies, retail pharmacies, and e-commerce. Hospital pharmacies control a substantial portion of the market due to the high demand for these syringes in inpatient care. However, the growing trend toward e-commerce arises from convenience and access, catering to increasing consumer demands.
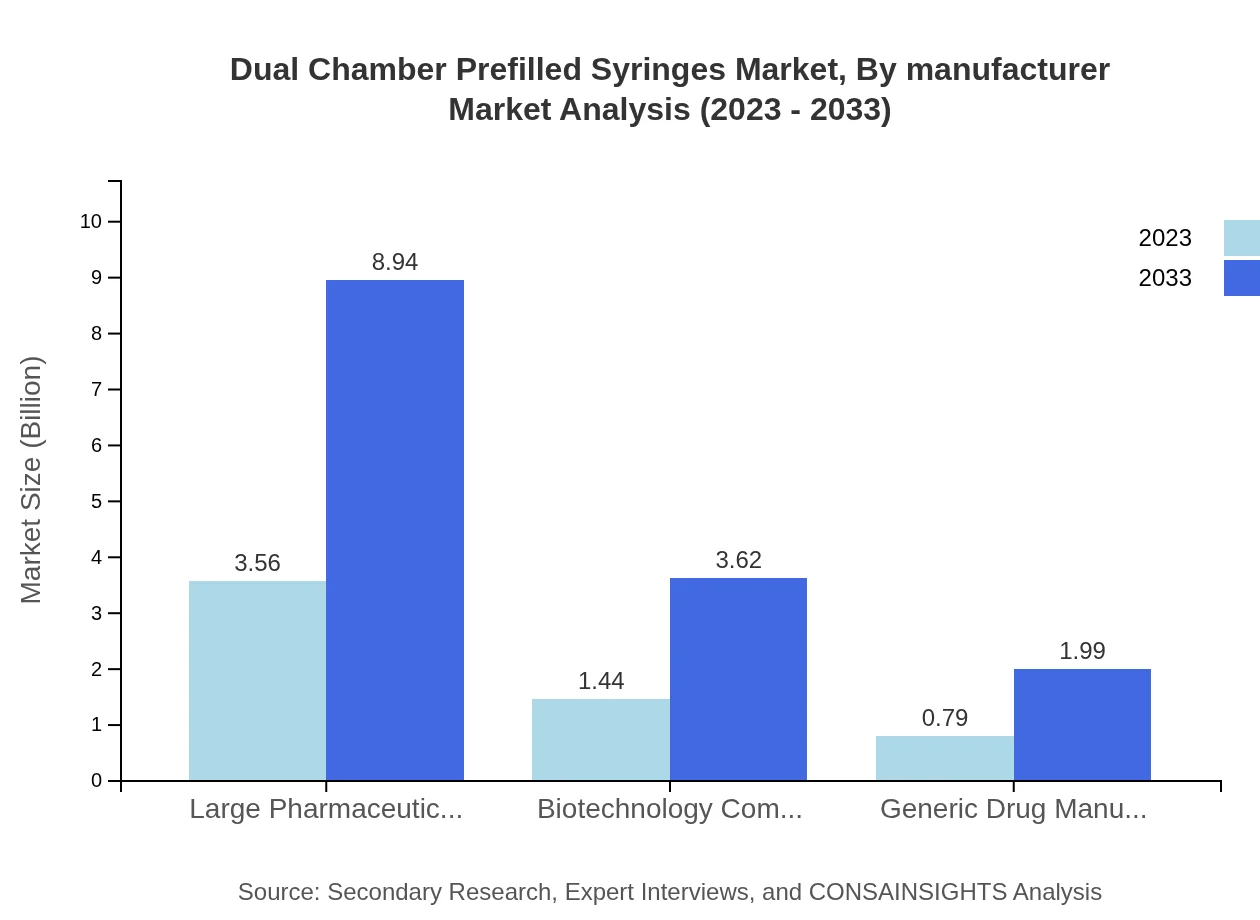
Dual Chamber Prefilled Syringes Market Analysis By Manufacturer
The manufacturer segment is characterized by large pharmaceutical companies, biotechnology companies, and generic drug manufacturers. Large players hold a dominant position due to their extensive R&D capabilities and established distribution networks. Meanwhile, biotechnology companies are essential for focusing on innovative therapies, while generic manufacturers ensure wider access to treatment.
Dual Chamber Prefilled Syringes Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Dual Chamber Prefilled Syringes Industry
BD (Becton, Dickinson and Company):
BD is a global leader in medical technology that develops and manufactures precision-engineered medical devices, including prefilled syringes, serving various therapeutic areas.Gerresheimer AG:
Gerresheimer AG specializes in high-quality pharmaceutical packaging and drug delivery systems. They manufacture a diverse range of prefilled syringes and are committed to addressing the needs of the biopharmaceutical industry.Schott AG:
Schott AG offers a broad spectrum of pharmaceutical packaging solutions, including Dual Chamber Prefilled Syringes. Their products are known for safety, efficiency, and compatibility with various drug formulations.West Pharmaceutical Services, Inc.:
West provides innovative drug delivery solutions, emphasizing prefilled syringes and combination products that enhance user safety and improve patient outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of dual Chamber Prefilled Syringes?
The global dual-chamber prefilled syringes market size was valued at approximately $5.8 billion in 2023. This market is anticipated to grow at a CAGR of 9.3% through to 2033, highlighting significant demand and expansion potential.
What are the key market players or companies in this dual Chamber Prefilled Syringes industry?
Key market players in the dual-chamber prefilled syringes industry include major pharmaceutical companies, biotechnology firms, and manufacturers focusing on innovative drug delivery systems. Their collaboration often enhances product development and market reach.
What are the primary factors driving the growth in the dual Chamber Prefilled Syringes industry?
The growth in the dual-chamber prefilled syringes industry is primarily driven by increased demand for injectable biologics, a growing focus on patient safety, and the need for convenient drug administration options in various healthcare settings.
Which region is the fastest Growing in the dual Chamber Prefilled Syringes?
The fastest-growing region in the dual-chamber prefilled syringes market is North America, expected to rise from $2.12 billion in 2023 to $5.32 billion by 2033, attributed to advanced healthcare infrastructure and a high prevalence of chronic diseases.
Does ConsaInsights provide customized market report data for the dual Chamber Prefilled Syringes industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the dual-chamber prefilled syringes industry, providing insights that align with unique business objectives, market strategies, and regional focus.
What deliverables can I expect from this dual Chamber Prefilled Syringes market research project?
Expected deliverables from the dual-chamber prefilled syringes market research project include comprehensive market analyses, trend forecasts, competitive landscape evaluations, and actionable insights into specific market segments and regional opportunities.
What are the market trends of dual Chamber Prefilled Syringes?
Key market trends for dual-chamber prefilled syringes include a rise in self-administration options, increased focus on patient-centric products, and innovation in syringe design aimed at enhancing safety and efficiency in drug delivery.