Duchenne Muscular Dystrophy Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: duchenne-muscular-dystrophy-therapeutics
Duchenne Muscular Dystrophy Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Duchenne Muscular Dystrophy (DMD) therapeutics market, covering market trends, size, and growth projections from 2023 to 2033. Insights into regional analysis, product segmentation, and leading companies are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
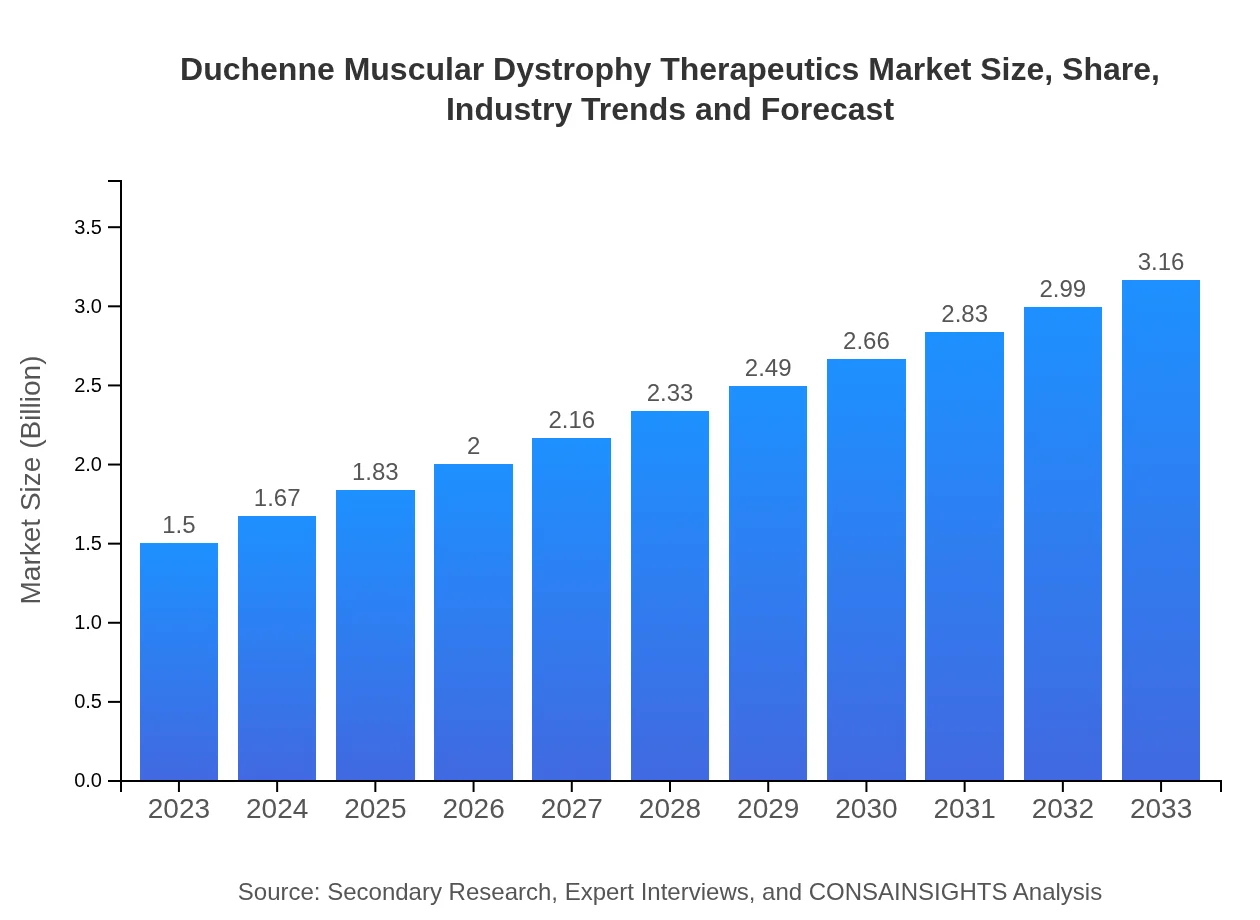
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $3.16 Billion |
| Top Companies | Sarepta Therapeutics, Pfizer , Boehringer Ingelheim, Catabasis Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Duchenne Muscular Dystrophy Therapeutics Market Overview
Customize Duchenne Muscular Dystrophy Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Duchenne Muscular Dystrophy Therapeutics market size, growth, and forecasts.
- ✔ Understand Duchenne Muscular Dystrophy Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Duchenne Muscular Dystrophy Therapeutics
What is the Market Size & CAGR of Duchenne Muscular Dystrophy Therapeutics market in 2023?
Duchenne Muscular Dystrophy Therapeutics Industry Analysis
Duchenne Muscular Dystrophy Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Duchenne Muscular Dystrophy Therapeutics Market Analysis Report by Region
Europe Duchenne Muscular Dystrophy Therapeutics Market Report:
Europe's DMD therapeutics market is set to increase significantly, with projections of $0.53 billion in 2023 growing to $1.12 billion by 2033. The region benefits from robust healthcare systems and a well-established pipeline of innovative therapies.Asia Pacific Duchenne Muscular Dystrophy Therapeutics Market Report:
The Asia Pacific region presents significant growth potential, expecting a market size growth from $0.26 billion in 2023 to $0.55 billion by 2033. The increase is driven by the rising prevalence of DMD, improving healthcare infrastructure, and greater investments in biotechnology.North America Duchenne Muscular Dystrophy Therapeutics Market Report:
North America remains the largest market for DMD therapeutics, with a projected rise from $0.50 billion in 2023 to $1.06 billion in 2033. This growth is fueled by a favorable regulatory environment, significant R&D investments, and the presence of leading pharmaceutical companies.South America Duchenne Muscular Dystrophy Therapeutics Market Report:
In South America, the market is anticipated to expand from $0.09 billion in 2023 to $0.19 billion by 2033, highlighting a growing recognition of DMD and efforts to enhance access to treatments, despite existing challenges such as economic instability.Middle East & Africa Duchenne Muscular Dystrophy Therapeutics Market Report:
The Middle East and Africa market is expected to grow from $0.11 billion in 2023 to $0.23 billion in 2033. This growth is spurred by improved access to healthcare and increasing awareness regarding genetic disorders, including DMD.Tell us your focus area and get a customized research report.
Duchenne Muscular Dystrophy Therapeutics Market Analysis By Therapeutic Approach
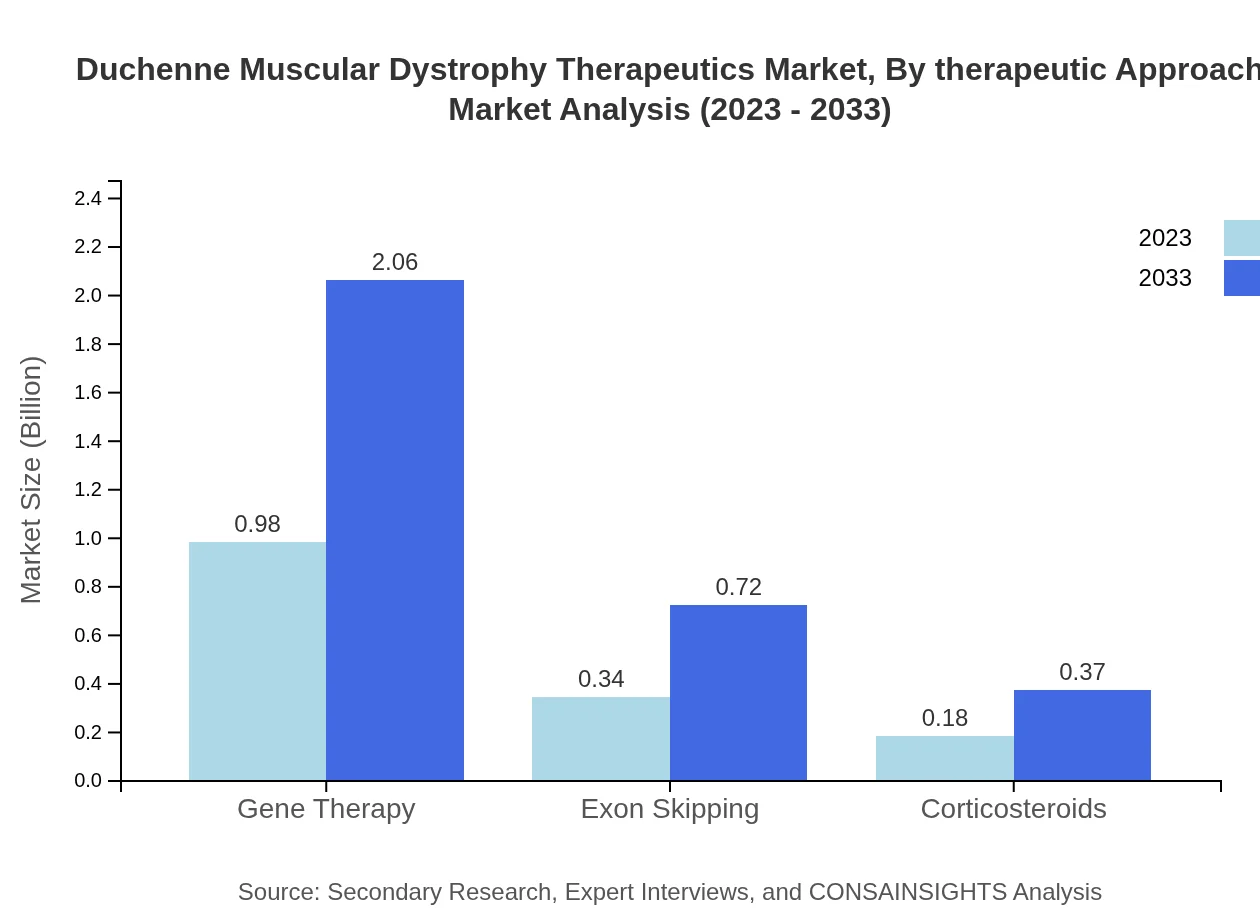
The therapeutic approach segment in the DMD market is dominated by muscle repair agents, which account for a market size of $1.30 billion in 2023, anticipated to rise to $2.73 billion by 2033. Anti-inflammatory drugs, while significant, contribute less, showcasing a need for more innovative therapies to address the multifaceted aspects of the disease.
Duchenne Muscular Dystrophy Therapeutics Market Analysis By Drug Type
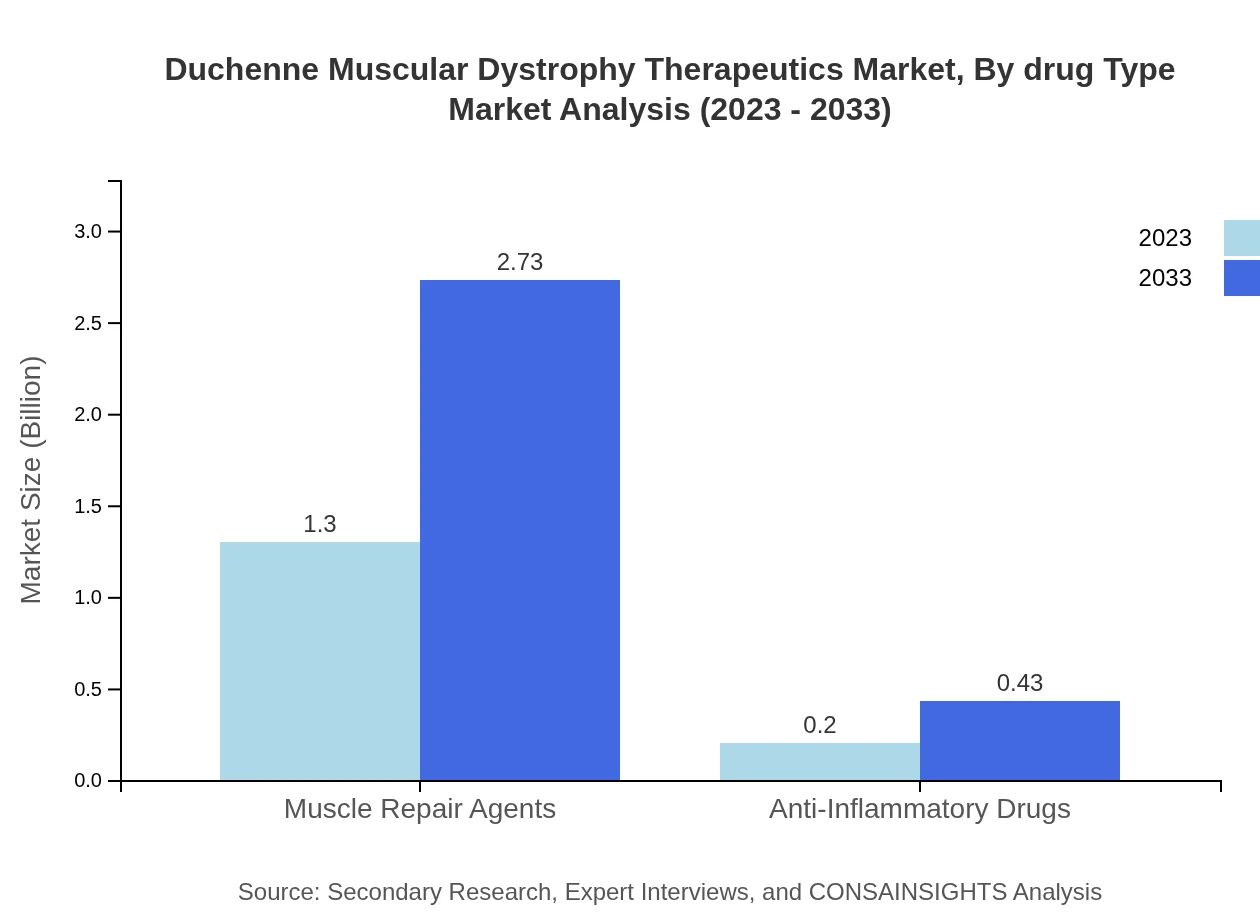
Within the drug type segmentation, muscle repair agents are crucial, showing a market size growth from $1.30 billion in 2023 to $2.73 billion by 2033. This segment reflects ongoing research and breakthroughs in the therapeutic pipeline aimed at repairing muscle tissues damaged by DMD.
Duchenne Muscular Dystrophy Therapeutics Market Analysis By Route Of Administration
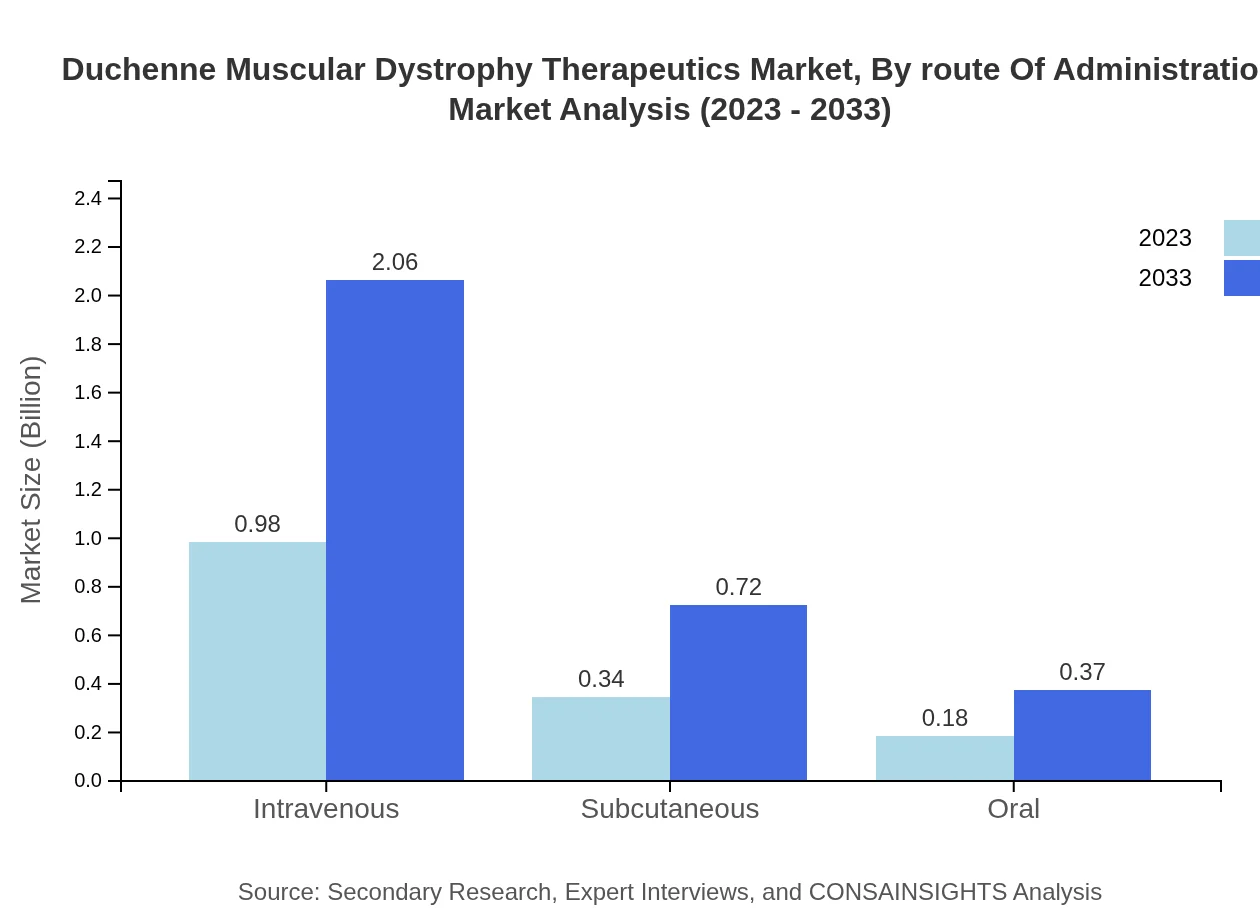
Intravenous administration leads the route of administration segment with a size of $0.98 billion in 2023, expected to increase to $2.06 billion by 2033. Subcutaneous and oral routes, while gaining traction, represent smaller shares of the market due to specific delivery challenges associated with gene therapies.
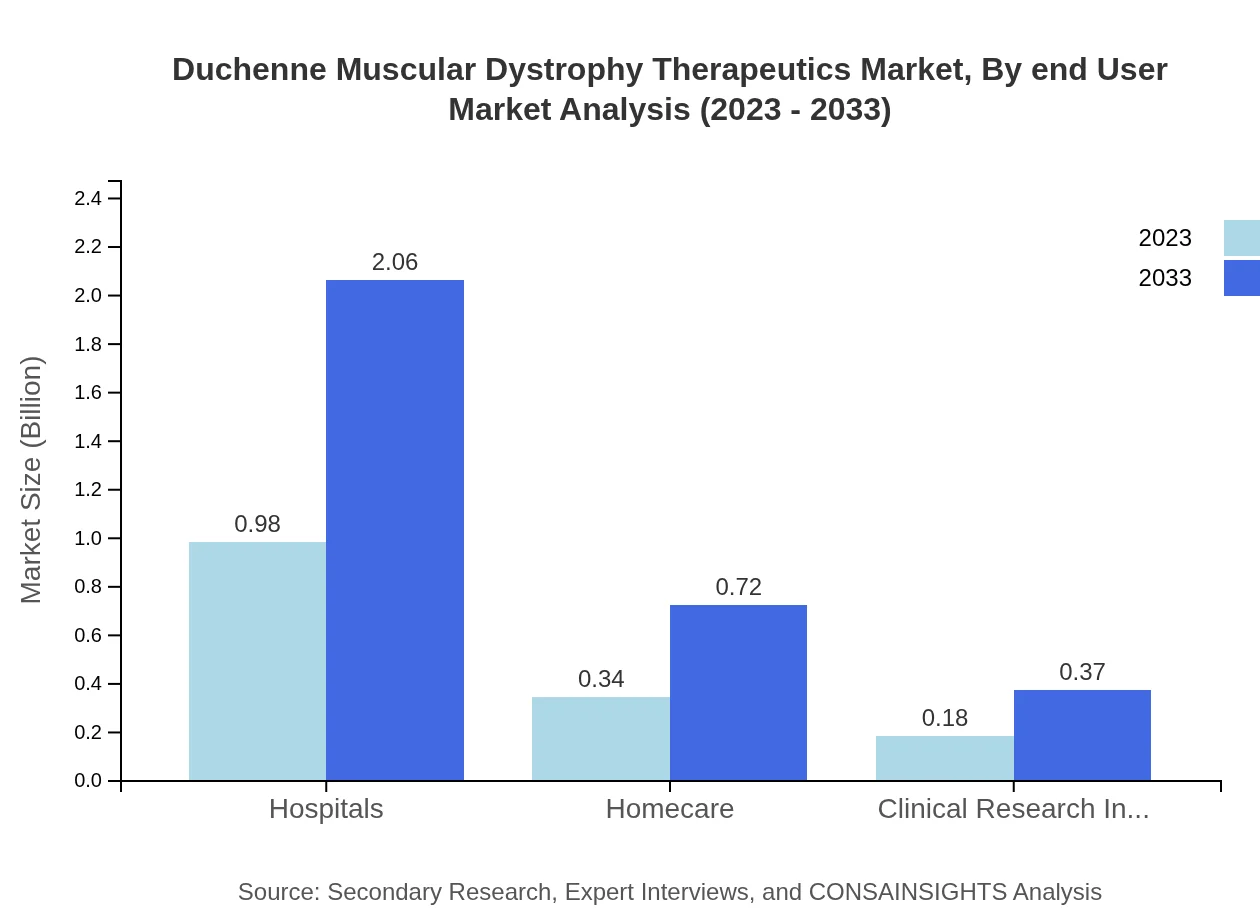
Duchenne Muscular Dystrophy Therapeutics Market Analysis By End User
Hospitals dominate the end-user segment, accounting for 65.38% of the market share. With a market size of $0.98 billion in 2023, it is projected to reach $2.06 billion by 2033. Homecare settings are also significant, reflecting a shift towards broader access to therapies beyond acute care.
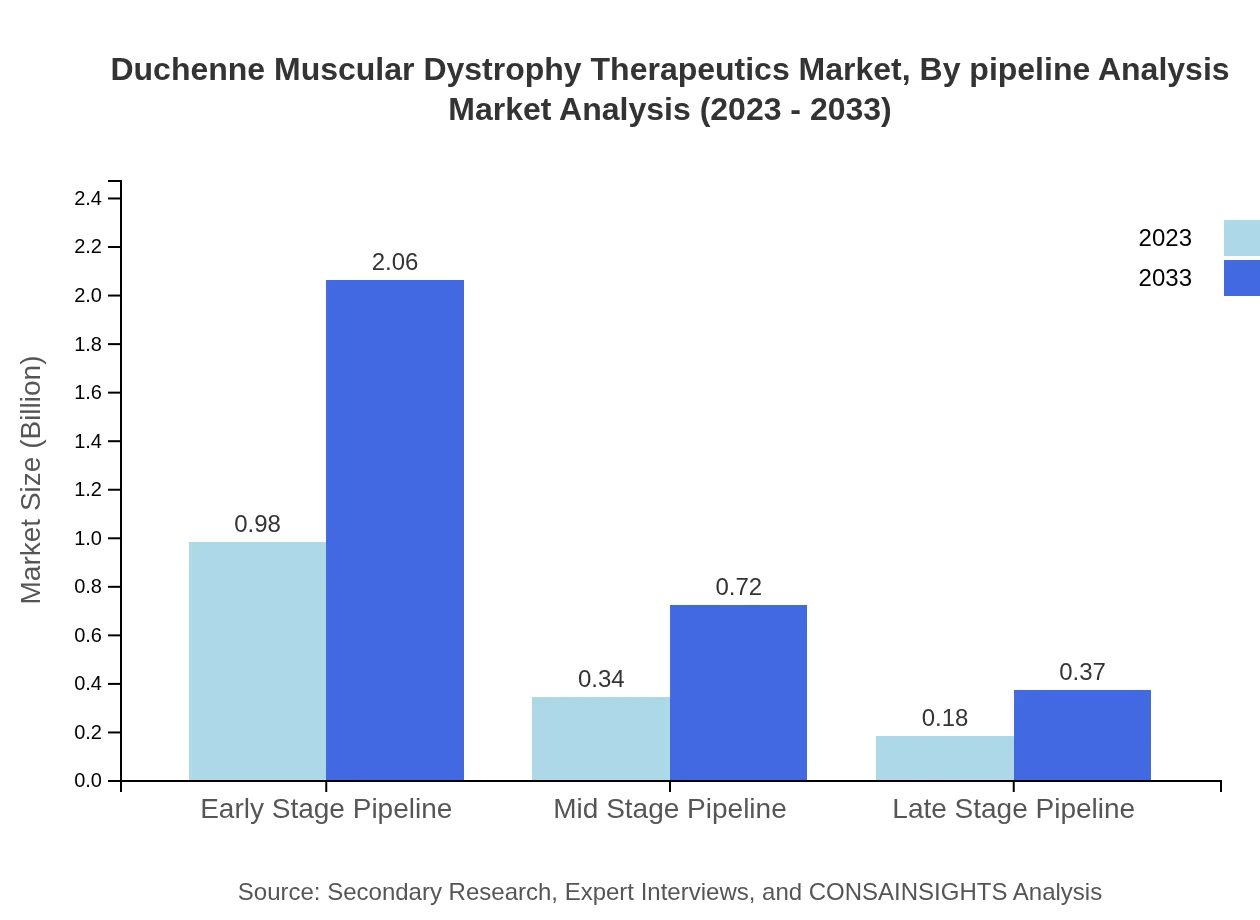
Duchenne Muscular Dystrophy Therapeutics Market Analysis By Pipeline Analysis
The pipeline analysis indicates that early-stage therapies constitute around 65.38% market share. As these therapies progress through the development stages, expected market entries will significantly impact future growth, specifically in gene-targeted approaches and novel delivery systems.
Duchenne Muscular Dystrophy Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Duchenne Muscular Dystrophy Therapeutics Industry
Sarepta Therapeutics:
Sarepta Therapeutics is a frontrunner in gene therapy for DMD, known for its innovative Exondys 51 treatment, targeting the underlying cause of DMD.Pfizer :
Pfizer's significant investments in DMD research have led to promising advancements in muscle repair therapies and genetic medicine.Boehringer Ingelheim:
Boehringer Ingelheim focuses on developing therapies incorporating gene therapy methodologies, aimed at preventing muscle degeneration in DMD patients.Catabasis Pharmaceuticals:
Catabasis Pharmaceuticals aims to develop therapies that enhance muscle function and systemic health in DMD patients through innovative drug formulations.We're grateful to work with incredible clients.









FAQs
What is the market size of duchenne Muscular Dystrophy Therapeutics?
The Duchenne Muscular Dystrophy Therapeutics market is currently valued at approximately $1.5 billion, with a projected compound annual growth rate (CAGR) of 7.5% through 2033. This growth highlights increasing investments in developed therapies and drug research.
What are the key market players or companies in this duchenne Muscular Dystrophy Therapeutics industry?
Key players in the Duchenne Muscular Dystrophy therapeutics industry include major pharmaceutical companies and biotechnology firms focusing on innovative treatment modalities, gene therapies, and supportive care solutions. These companies are pivotal in driving advancements in clinical research and product development.
What are the primary factors driving the growth in the duchenne Muscular Dystrophy Therapeutics industry?
Growth within the Duchenne Muscular Dystrophy therapeutics sector is fueled by increased awareness, advancements in genetic research, regulatory support for innovative therapies, and the rising prevalence of the condition among children, prompting urgent demand for effective treatment options.
Which region is the fastest Growing in the duchenne Muscular Dystrophy Therapeutics?
The fastest-growing region for Duchenne Muscular Dystrophy therapeutics is North America, projected to expand from $0.50 billion in 2023 to $1.06 billion by 2033, spurred by robust healthcare infrastructure, research, and investment in biopharmaceuticals.
Does ConsaInsights provide customized market report data for the duchenne Muscular Dystrophy Therapeutics industry?
Yes, ConsaInsights offers tailored market reports and data specific to Duchenne Muscular Dystrophy therapeutics. Clients can request customized insights that cater to unique business needs, market conditions, and emerging trends in the sector.
What deliverables can I expect from this duchenne Muscular Dystrophy Therapeutics market research project?
Deliverables from a Duchenne Muscular Dystrophy therapeutics market research project typically include comprehensive reports, data analysis, market forecasts, competitive landscape overviews, and valuable insights into industry trends, tailored to client specifications.
What are the market trends of duchenne Muscular Dystrophy Therapeutics?
Trends in the Duchenne Muscular Dystrophy therapeutics market include a focus on gene therapy advancements, increased investment in early-stage pipelines, rising adoption of muscle repair agents, and a shift towards personalized medicine, enhancing treatment efficacy.