Duchenne Muscular Dystrophy Treatment Market Report
Published Date: 31 January 2026 | Report Code: duchenne-muscular-dystrophy-treatment
Duchenne Muscular Dystrophy Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Duchenne Muscular Dystrophy (DMD) treatment market from 2023 to 2033, highlighting market dynamics, regional insights, segmentation, technological impacts, and forecasts for growth and challenges ahead.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
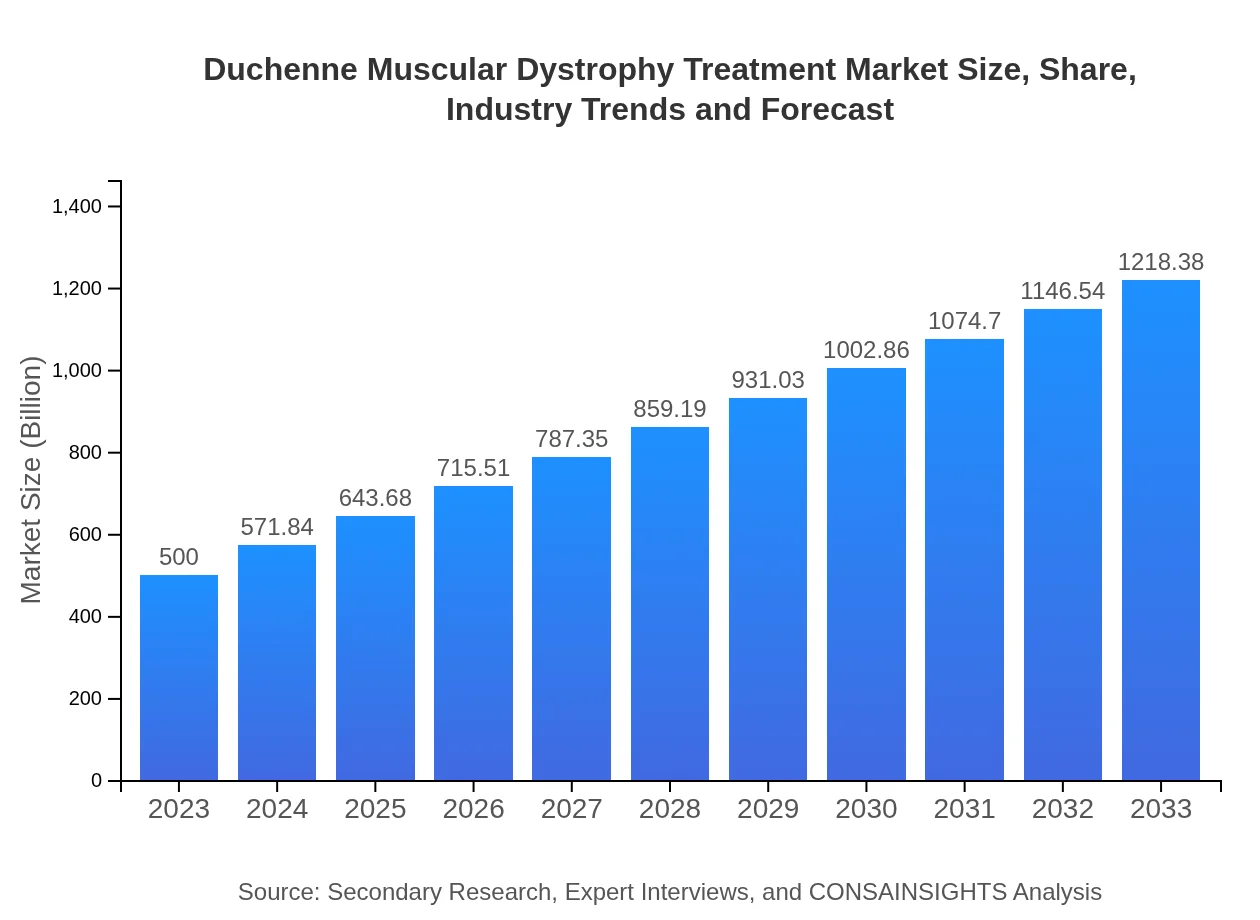
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 9% |
| 2033 Market Size | $1218.38 Million |
| Top Companies | Sarepta Therapeutics, Pfizer, Inc., Solid Biosciences, Vertex Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Duchenne Muscular Dystrophy Treatment Market Overview
Customize Duchenne Muscular Dystrophy Treatment Market Report market research report
- ✔ Get in-depth analysis of Duchenne Muscular Dystrophy Treatment market size, growth, and forecasts.
- ✔ Understand Duchenne Muscular Dystrophy Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Duchenne Muscular Dystrophy Treatment
What is the Market Size & CAGR of Duchenne Muscular Dystrophy Treatment market in 2023?
Duchenne Muscular Dystrophy Treatment Industry Analysis
Duchenne Muscular Dystrophy Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Duchenne Muscular Dystrophy Treatment Market Analysis Report by Region
Europe Duchenne Muscular Dystrophy Treatment Market Report:
The European market, valued at $168.60 million in 2023, will see growth to $410.84 million by 2033, fueled by strong regulatory frameworks supporting innovative treatments and widespread availability of therapies.Asia Pacific Duchenne Muscular Dystrophy Treatment Market Report:
In Asia Pacific, the DMD treatment market is expected to grow from $90.15 million in 2023 to $219.67 million in 2033, driven by improving healthcare infrastructure and increased awareness of rare diseases.North America Duchenne Muscular Dystrophy Treatment Market Report:
North America, being a leader in DMD research, holds a market value of $171.05 million in 2023, projected to grow to $416.81 million by 2033 due to high investment in R&D and established healthcare systems.South America Duchenne Muscular Dystrophy Treatment Market Report:
The South American market, starting at $41.15 million in 2023, will reach $100.27 million by 2033. Growth is supported by increasing collaboration among health ministries and organizations aimed at addressing rare diseases.Middle East & Africa Duchenne Muscular Dystrophy Treatment Market Report:
The Middle East and Africa region begins at $29.05 million in 2023, forecast to reach $70.79 million by 2033, with growth driven by improving healthcare access and regulatory support for new treatments.Tell us your focus area and get a customized research report.
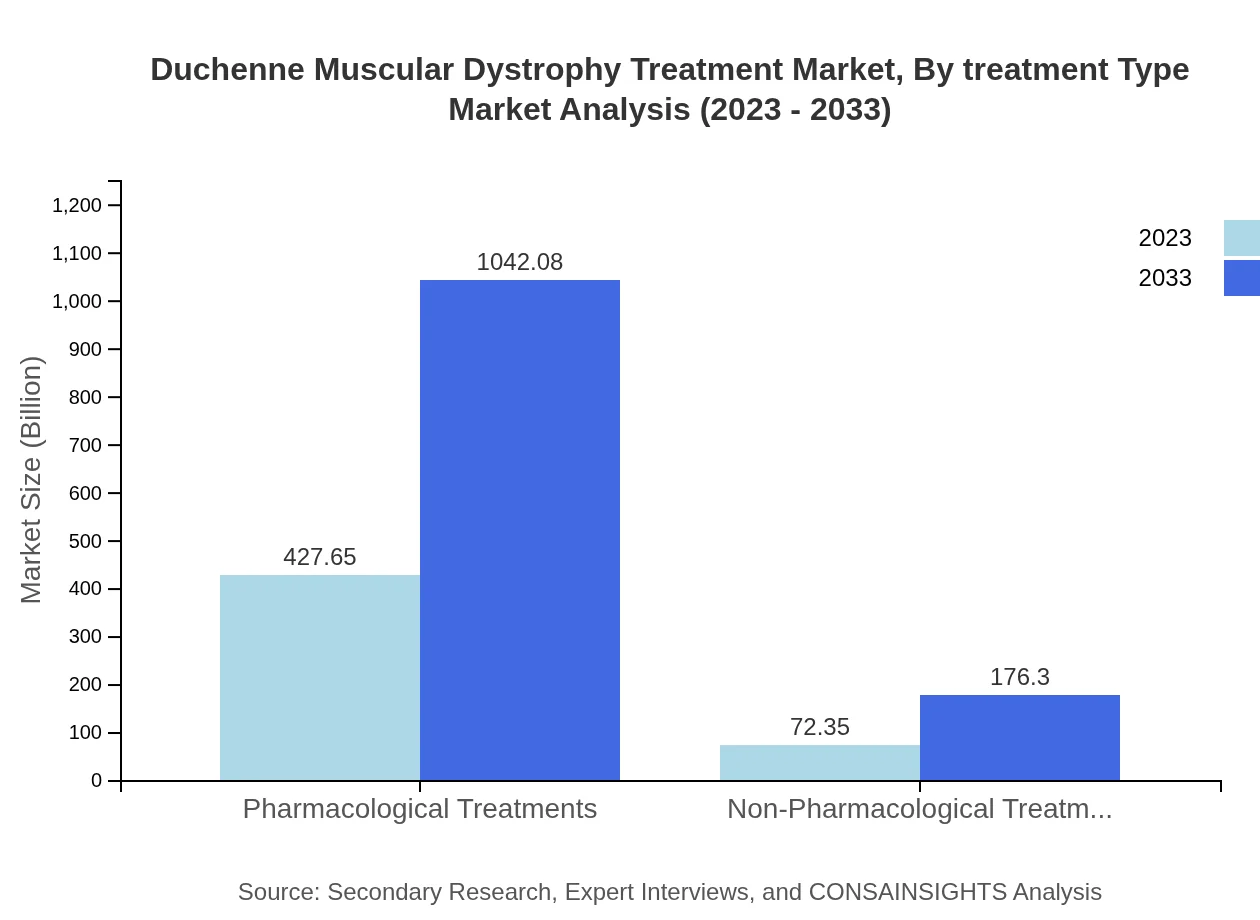
Duchenne Muscular Dystrophy Treatment Market Analysis By Treatment Type
Pharmacological treatments currently dominate the DMD treatment market, accounting for approximately 85.53% of the market share in 2023, with a market size of $427.65 million. This segment is expected to reach $1 billion by 2033. Non-pharmacological treatments, though currently at $72.35 million (14.47% share), are gaining traction as essential complementary therapies for DMD management.
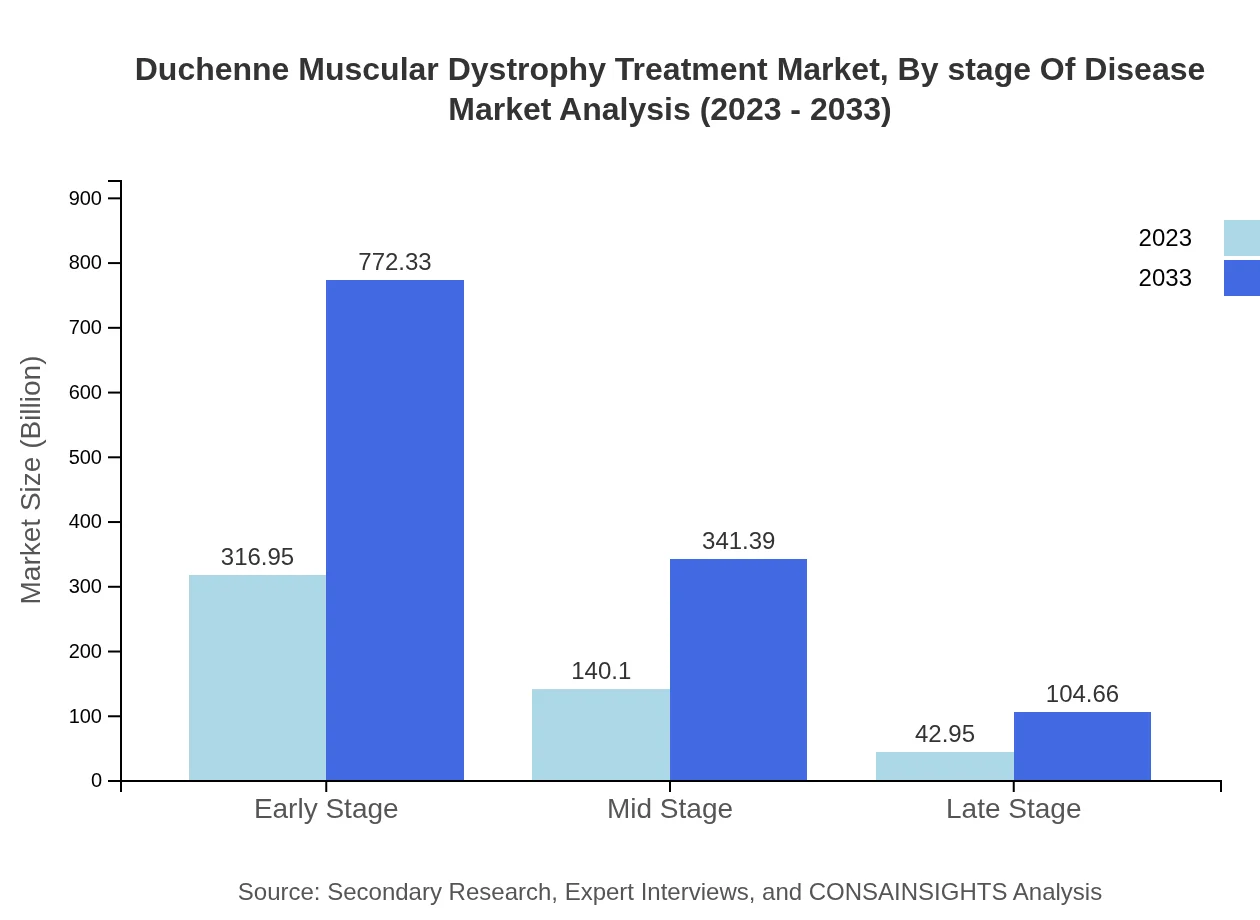
Duchenne Muscular Dystrophy Treatment Market Analysis By Stage Of Disease
The majority of the market is represented by early (63.39% share) and mid-stage patients (28.02% share), with respective market sizes of $316.95 million and $140.10 million projected to grow substantially by 2033. Late-stage patients continue to represent a smaller segment but remain critical for the continuous care aspect.
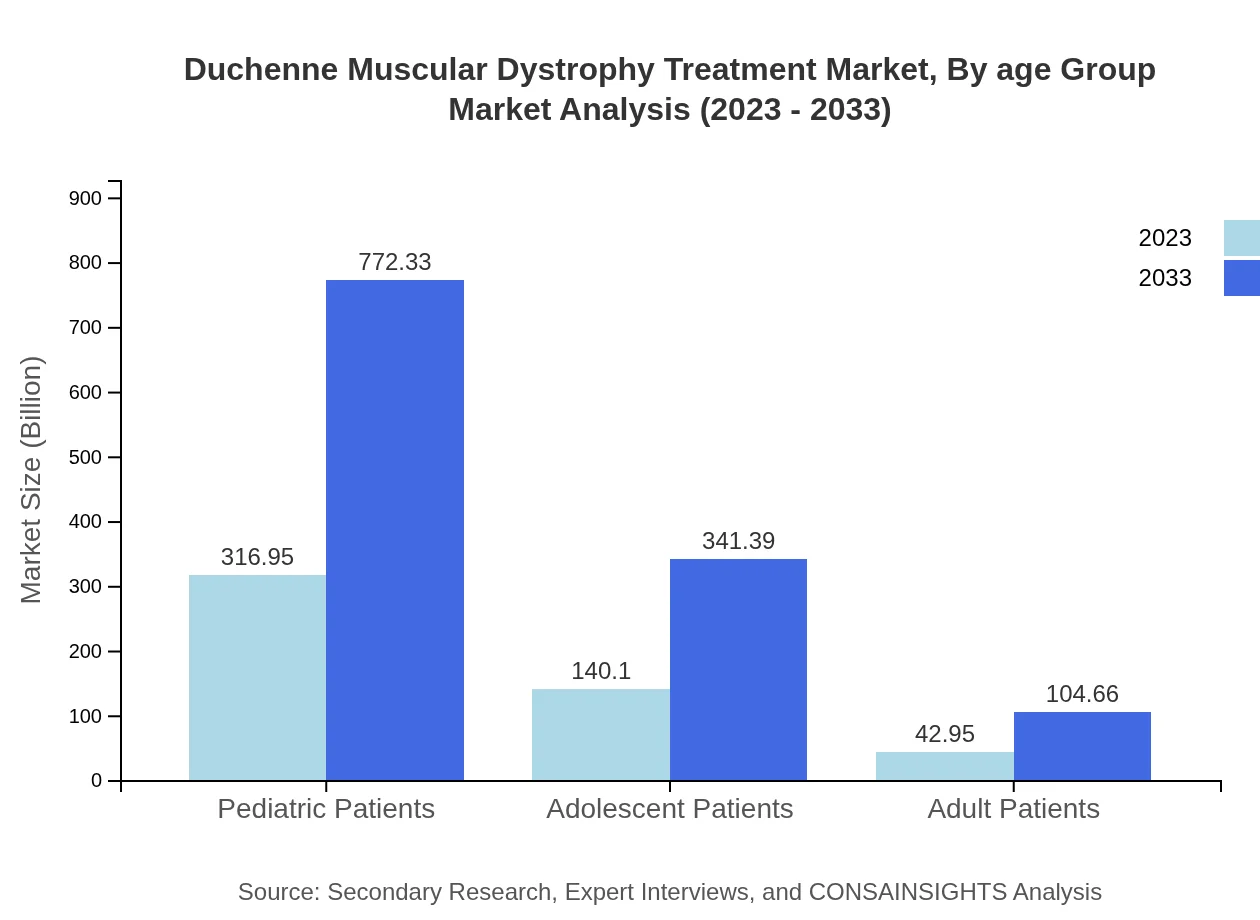
Duchenne Muscular Dystrophy Treatment Market Analysis By Age Group
The pediatric segment is the largest, with a market size of $316.95 million for pediatric patients, reflecting a share of 63.39% in 2023. The adolescent segment, although smaller at $140.10 million (28.02%), shows potential for growth, driven by the transition of care as patients age.
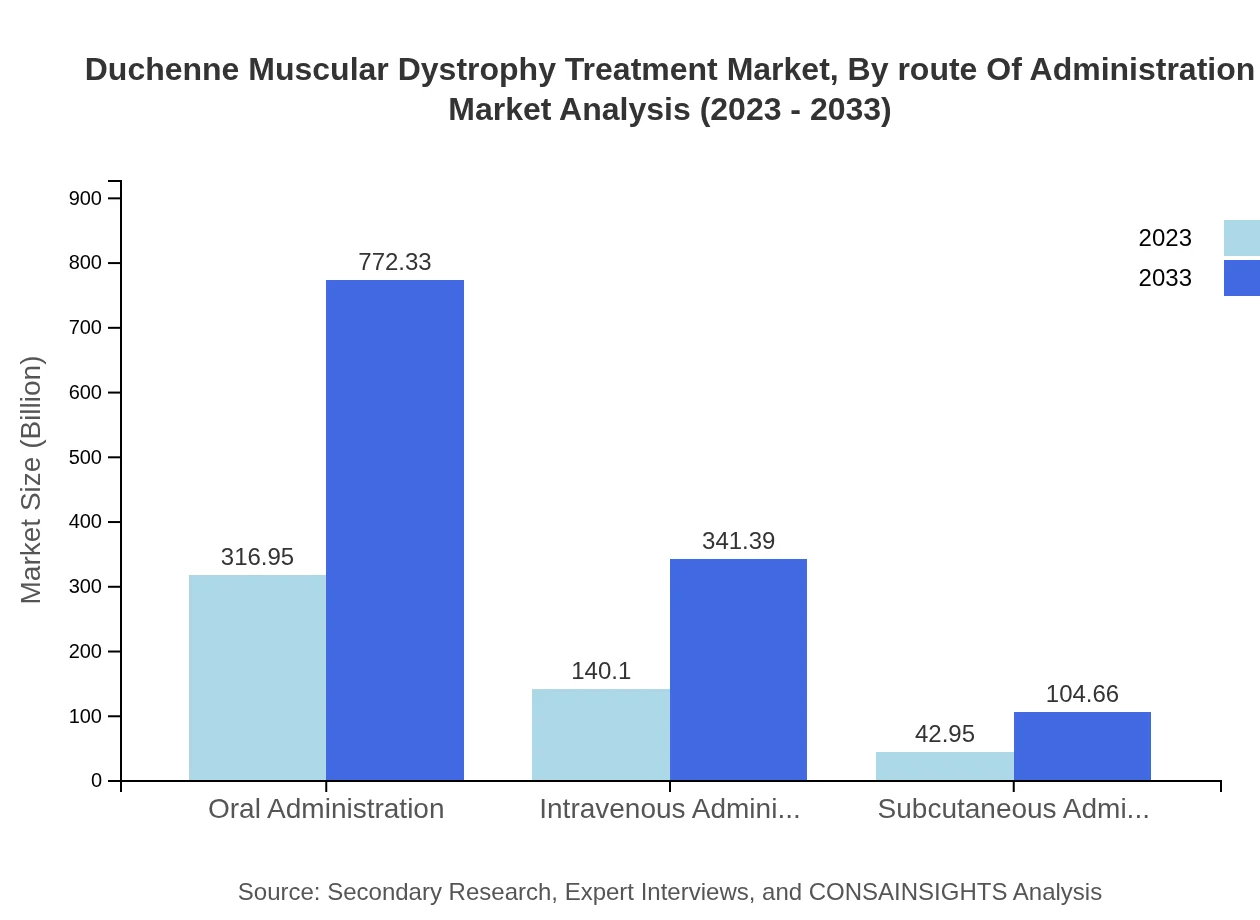
Duchenne Muscular Dystrophy Treatment Market Analysis By Route Of Administration
Oral administration dominates this segment, accounting for over 63% of the market share, valued at $316.95 million in 2023. Intravenous and subcutaneous methods, while less common, are important for certain therapies, showing strong anticipated growth in the coming years.
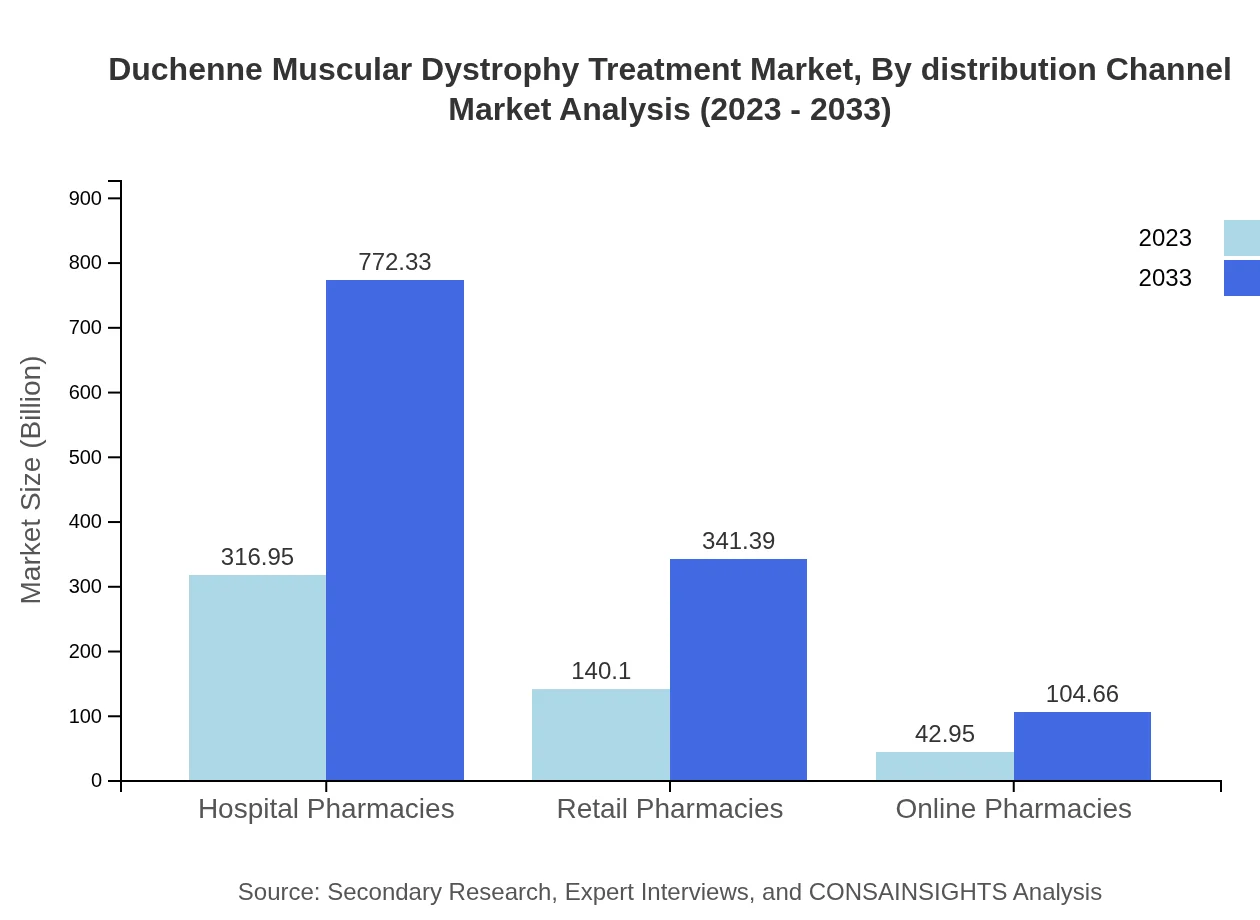
Duchenne Muscular Dystrophy Treatment Market Analysis By Distribution Channel
Hospital pharmacies constitute a significant share of the distribution channel for DMD treatments, at 63.39%, with a market size of $316.95 million. Retail and online pharmacies, while smaller, are critical for wider access to medications and show growth potential as patient needs evolve.
Duchenne Muscular Dystrophy Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Duchenne Muscular Dystrophy Treatment Industry
Sarepta Therapeutics:
A pioneer in DMD therapies with a focus on gene and RNA-targeted treatments. The company leads the market with innovative products in clinical trials and successful launches.Pfizer, Inc.:
A significant player in the biopharmaceutical industry, Pfizer is involved in DMD drug development, contributing to comprehensive treatment options through research and partnerships.Solid Biosciences:
Focusing on gene therapy for DMD, Solid Biosciences employs innovative methods to create potential treatments designed to address the underlying causes of DMD.Vertex Pharmaceuticals:
Engaged in developing therapies for genetic diseases, Vertex aims to leverage its expertise towards advancing treatments for DMD.We're grateful to work with incredible clients.









FAQs
What is the market size of Duchenne Muscular Dystrophy treatment?
The Duchenne Muscular Dystrophy treatment market is projected to grow from approximately $500 million in 2023, with a CAGR of 9%, indicating robust expansion as advancements in therapies are made until 2033.
What are the key market players or companies in this Duchenne Muscular Dystrophy treatment industry?
Key players in the Duchenne Muscular Dystrophy treatment market include large pharmaceutical companies, biotech firms, and specialized manufacturers. These companies contribute significantly to research, development, and marketing treatments tailored for DMD.
What are the primary factors driving the growth in the Duchenne Muscular Dystrophy treatment industry?
Factors driving market growth include increased prevalence rates of DMD, rising awareness, advancements in medical technologies, and robust investment in research and development of innovative treatments enhancing quality of life.
Which region is the fastest Growing in the Duchenne Muscular Dystrophy treatment market?
The Asia Pacific region is expected to demonstrate significant growth, expanding from $90.15 million in 2023 to $219.67 million by 2033, reflecting a growing focus on healthcare innovations and accessibility.
Does ConsaInsights provide customized market report data for the Duchenne Muscular Dystrophy treatment industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the Duchenne Muscular Dystrophy treatment sector, providing insights relevant to particular demographics or therapeutic approaches.
What deliverables can I expect from this Duchenne Muscular Dystrophy treatment market research project?
Deliverables include comprehensive market reports, trend analysis, competitive landscape assessments, regional market size breakdowns, and insights into emerging treatment modalities tailored to Duchenne Muscular Dystrophy.
What are the market trends of Duchenne Muscular Dystrophy treatment?
Current trends encompass an increase in gene therapy research, growth in digital health solutions for patient monitoring, and expanding patient access to therapies, driving innovation and improved outcomes in DMD care.