Eclinical Solutions Market Report
Published Date: 31 January 2026 | Report Code: eclinical-solutions
Eclinical Solutions Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Eclinical Solutions market, covering insights into market size, growth trends, key regional analyses, segmentation, and leading companies. It forecasts the market from 2023 to 2033, highlighting opportunities and challenges.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
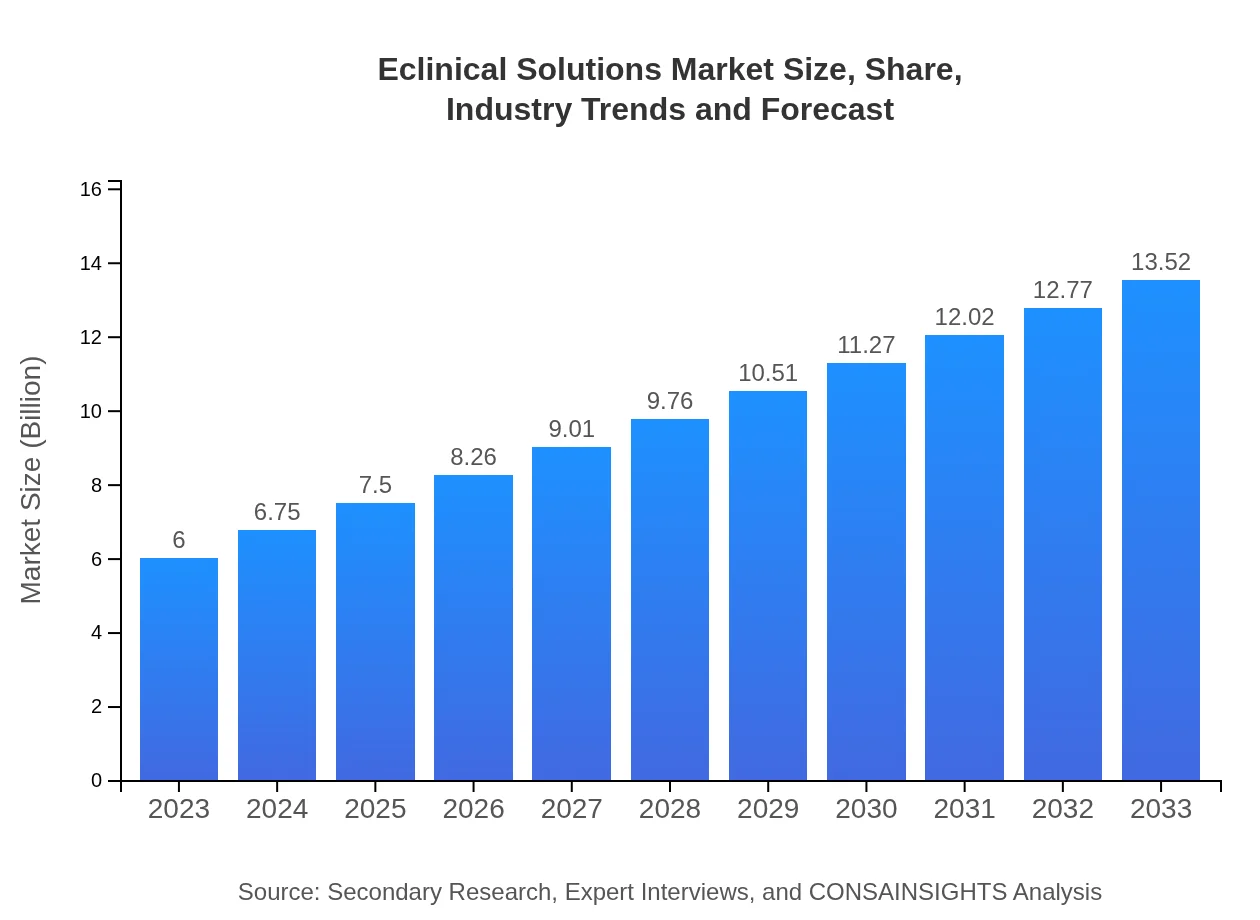
| 2023 Market Size | $6.00 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $13.52 Billion |
| Top Companies | Medidata Solutions, Oracle Health Sciences, Veeva Systems, ComplySci |
| Last Modified Date | 31 January 2026 |
Eclinical Solutions Market Overview
Customize Eclinical Solutions Market Report market research report
- ✔ Get in-depth analysis of Eclinical Solutions market size, growth, and forecasts.
- ✔ Understand Eclinical Solutions's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Eclinical Solutions
What is the Market Size & CAGR of Eclinical Solutions market in 2023?
Eclinical Solutions Industry Analysis
Eclinical Solutions Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Eclinical Solutions Market Analysis Report by Region
Europe Eclinical Solutions Market Report:
The European market for Eclinical Solutions, valued at USD 2.07 billion in 2023, is forecasted to grow to USD 4.67 billion by 2033. The region's strong regulatory framework and emphasis on innovative health solutions are key growth drivers.Asia Pacific Eclinical Solutions Market Report:
In the Asia Pacific region, the Eclinical Solutions market was valued at USD 1.03 billion in 2023, with expectations to reach USD 2.33 billion by 2033. Factors such as increasing R&D spending, a growing number of clinical trials, and supportive government initiatives are driving market growth.North America Eclinical Solutions Market Report:
In North America, a leading region with a market size of USD 2.04 billion in 2023, the Eclinical Solutions market is anticipated to grow to USD 4.60 billion by 2033, primarily due to the presence of key market players and substantial R&D investments.South America Eclinical Solutions Market Report:
The South American Eclinical Solutions market, valued at USD 0.46 billion in 2023, is projected to reach USD 1.04 billion by 2033. Economic growth and investment in healthcare infrastructure are key to its expansion.Middle East & Africa Eclinical Solutions Market Report:
The Eclinical Solutions market in the Middle East and Africa is smaller, currently at USD 0.40 billion in 2023, projected to grow to USD 0.90 billion by 2033, influenced by improving healthcare infrastructure and an increasing number of clinical trials.Tell us your focus area and get a customized research report.
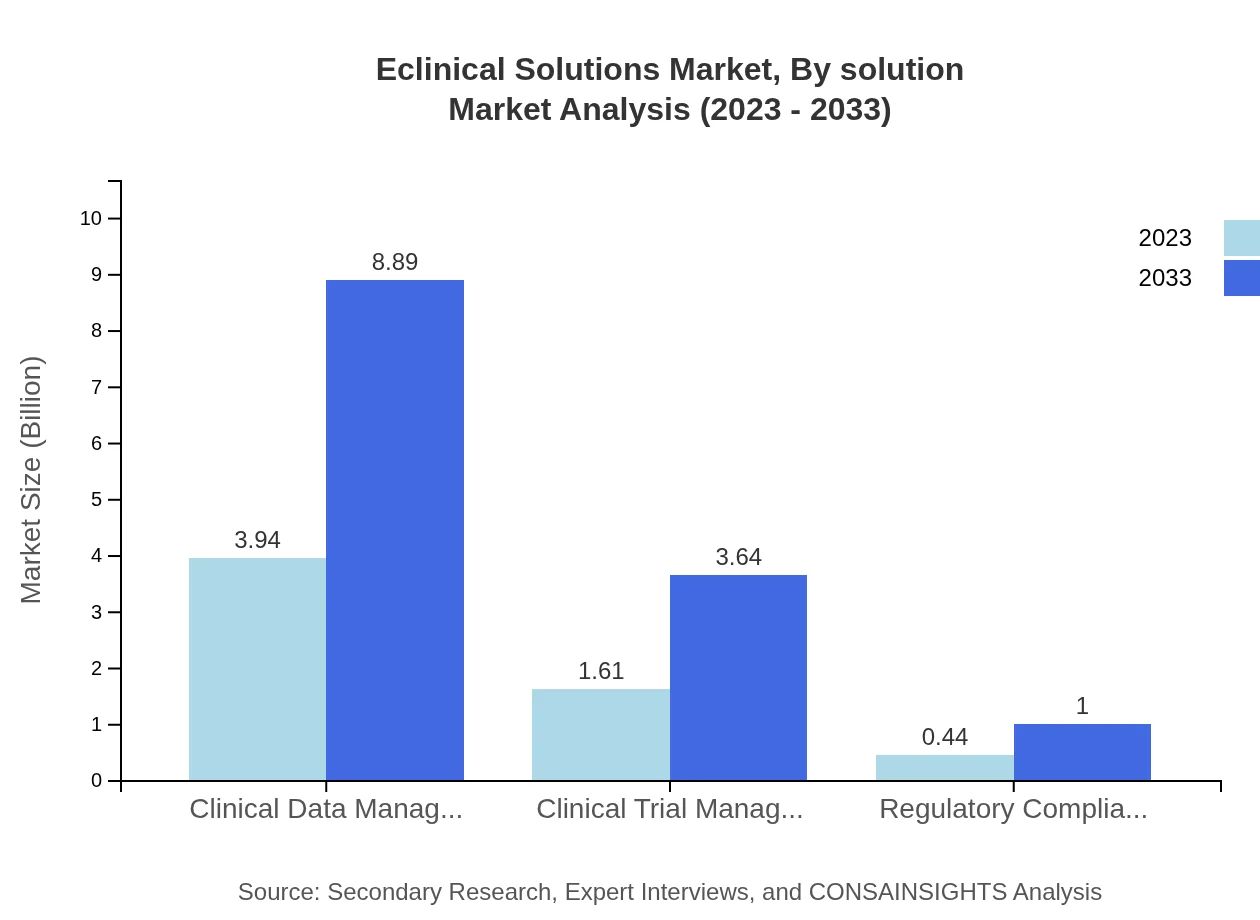
Eclinical Solutions Market Analysis By Solution
The market is predominantly driven by Cloud-Based Solutions, valued at USD 4.90 billion in 2023 and set to reach USD 11.06 billion by 2033, making up 81.74% of the share. Clinical Trial Management systems and Regulatory Compliance Solutions also hold significant positions, contributing notably to the comprehensive development of eclinical solutions.
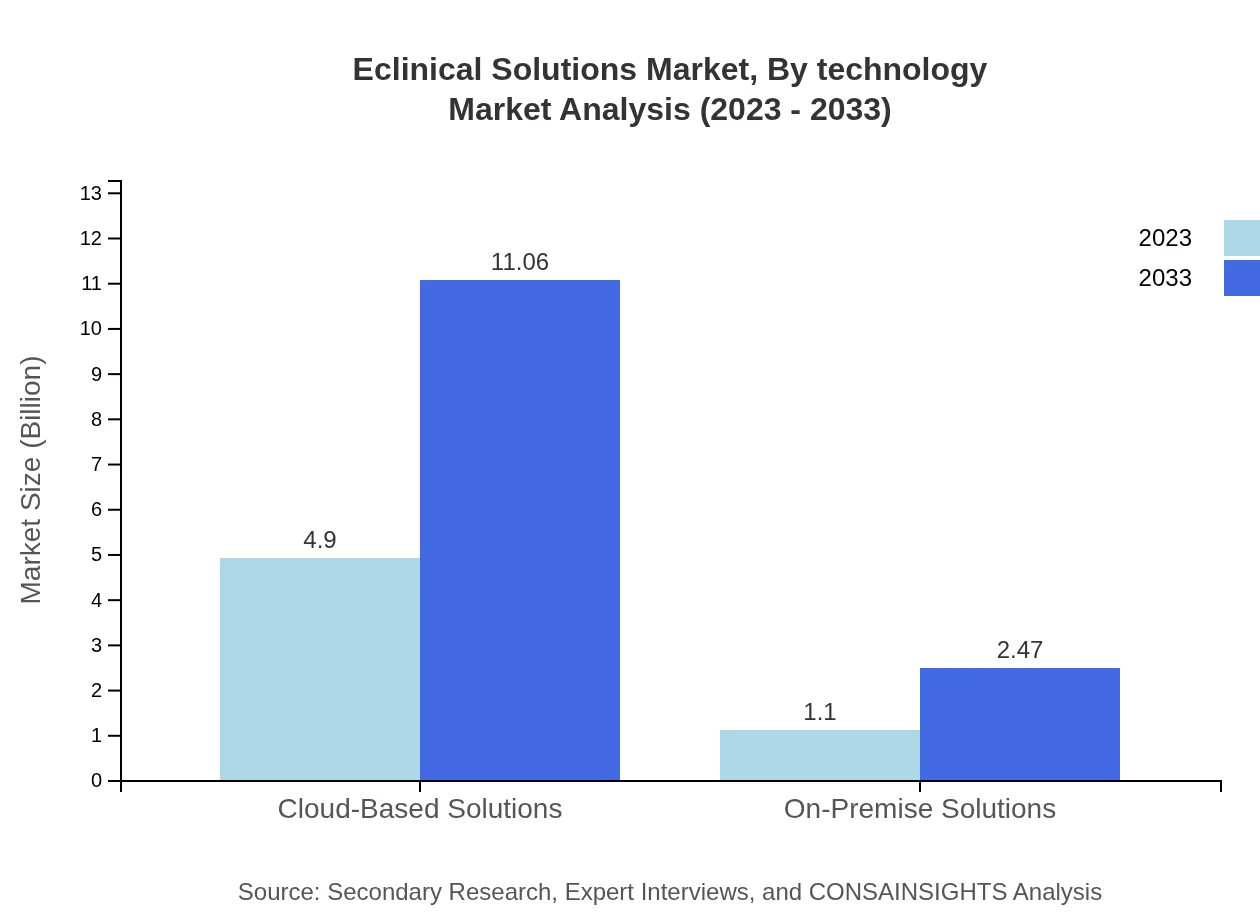
Eclinical Solutions Market Analysis By Technology
Technological advancements such as AI integration, machine learning, and cloud-based platforms are redefining the industry landscape. The focus is shifting towards incorporating real-time analytics and enhancing data security measures, critical for compliant trial management.
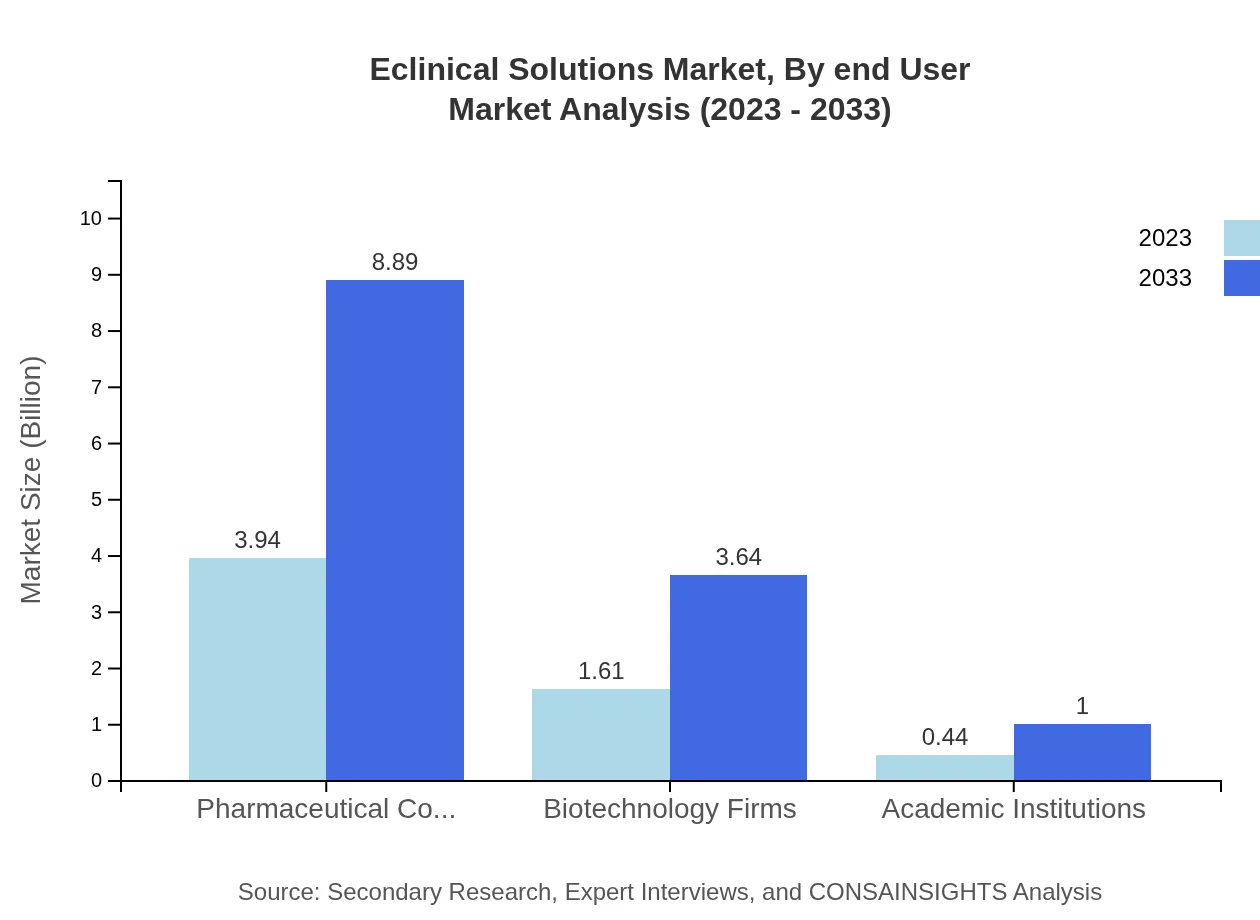
Eclinical Solutions Market Analysis By End User
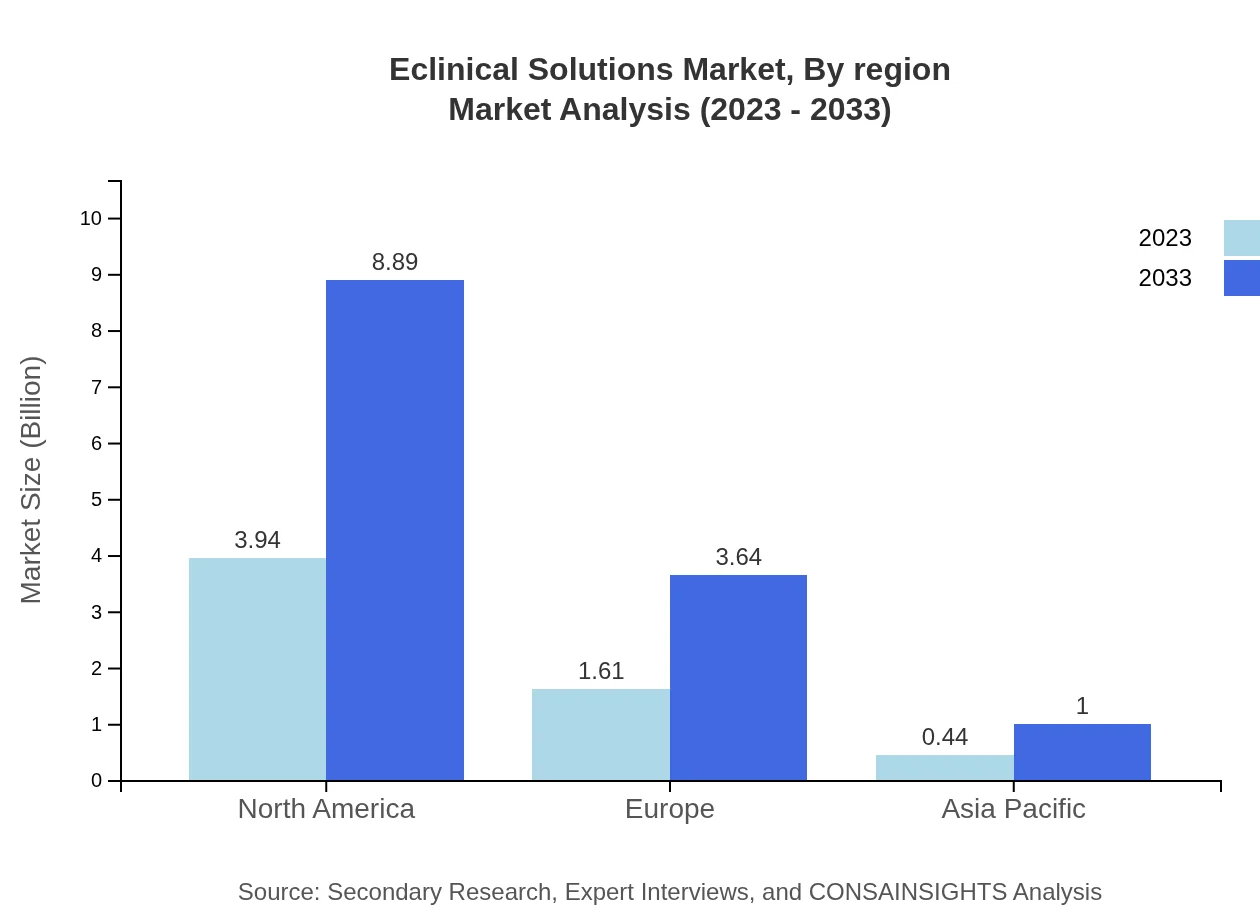
Pharmaceutical Companies lead the market, currently valued at USD 3.94 billion (2023) and anticipated to reach USD 8.89 billion by 2033, followed by Biotechnology Firms and Academic Institutions, representing substantial user bases that drive demand for Eclinical Solutions.
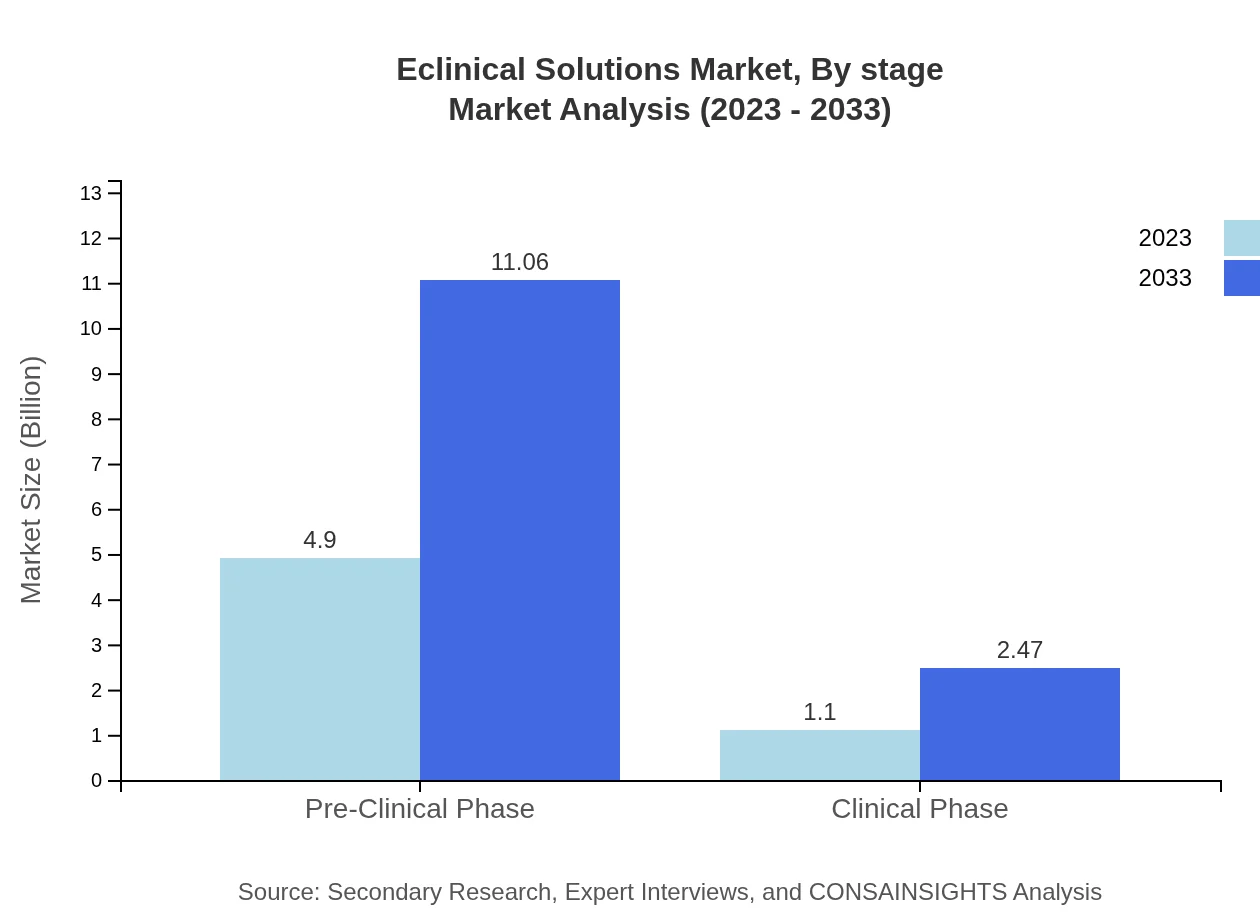
Eclinical Solutions Market Analysis By Stage
Segmenting by clinical stage, the Pre-Clinical Phase commands a significant portion of the market, valued at USD 4.90 billion in 2023 and expected to reach USD 11.06 billion by 2033. The Clinical Phase, while smaller, represents a growing area of focus as trials progress.
Eclinical Solutions Market Analysis By Region
In-depth regional analyses reveal that North America maintains a dominant market position, driven by advanced technology adoption and robust research frameworks, while Asia Pacific demonstrates rapid growth potential due to increasing investment in clinical trials.
Eclinical Solutions Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Eclinical Solutions Industry
Medidata Solutions:
Medidata Solutions is a leader in cloud-based solutions for clinical research, offering a comprehensive platform that supports the entire clinical trial lifecycle, thereby enhancing data integrity and operational efficiency.Oracle Health Sciences:
Oracle Health Sciences provides advanced solutions that support clinical development, including CTMS and EDC, enabling organizations to streamline operations while ensuring compliance with regulations.Veeva Systems:
Veeva Systems specializes in cloud software for the global life sciences industry, with a strong focus on providing solutions that manage clinical data efficiently, thereby improving the speed and quality of trials.ComplySci:
ComplySci focuses on compliance solutions for the financial industry, providing regulatory insights and solutions that can be pertinent to clinical trials in controlled environments.We're grateful to work with incredible clients.









FAQs
What is the market size of eclinical Solutions?
The eClinical Solutions market is currently valued at $6 billion in 2023, with a projected growth rate of 8.2% CAGR. By 2033, it is anticipated to reach substantial expansion, reflecting increasing demand in clinical trials and healthcare innovation.
What are the key market players or companies in this eclinical Solutions industry?
The eClinical Solutions industry has key players including major pharmaceutical companies and biotechnology firms. These entities participate actively, driving innovations and solutions that shape industry standards and expand market reach.
What are the primary factors driving the growth in the eclinical Solutions industry?
Growth in the eClinical Solutions industry is driven by rising demand for efficient clinical trials, technological advancements in data management, regulatory pressures, and increasing investments in healthcare and pharmaceutical developments.
Which region is the fastest Growing in the eclinical Solutions?
The fastest-growing region in the eClinical Solutions market is Europe, expected to increase from $2.07 billion in 2023 to $4.67 billion by 2033. North America follows closely, highlighting robust growth strategies in clinical innovations.
Does ConsaInsights provide customized market report data for the eclinical Solutions industry?
Yes, ConsaInsights offers customized market report data to cater to specific business needs in the eClinical Solutions industry, ensuring tailored insights that align with client requirements.
What deliverables can I expect from this eclinical Solutions market research project?
Deliverables from the eClinical Solutions market research project typically include comprehensive reports, statistical analysis, trend forecasts, and actionable insights to inform strategic decision making affecting market entry.
What are the market trends of eclinical Solutions?
Current market trends in the eClinical Solutions sector include an increasing shift towards cloud-based solutions, enhanced focus on patient-centric trials, and greater integration of AI-driven analytics to optimize clinical pathways.