Efficacy Testing Market Report
Published Date: 31 January 2026 | Report Code: efficacy-testing
Efficacy Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Efficacy Testing market from 2023 to 2033, including insights on market size, growth rate, segments, regional analysis, industry trends, and key players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
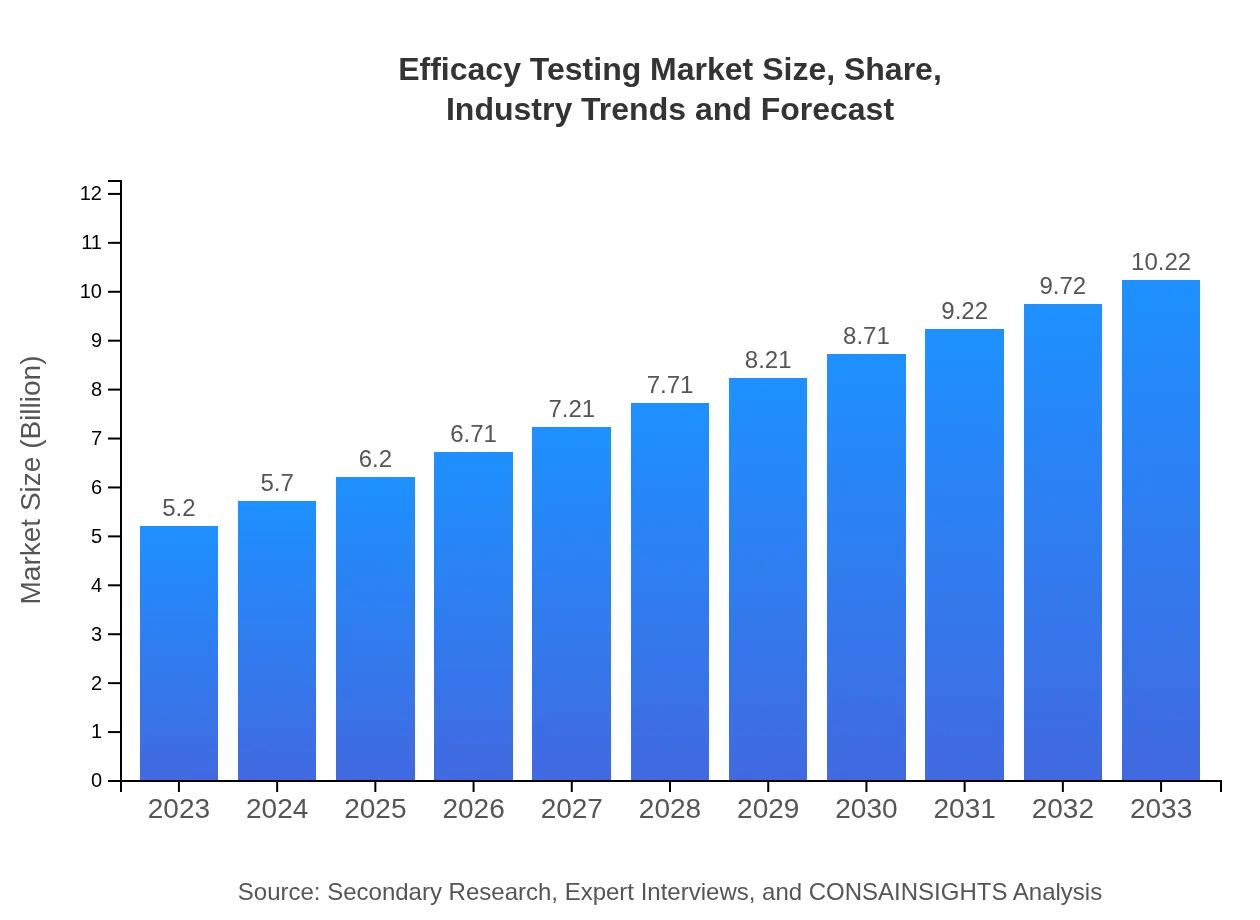
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Covance, Charles River Laboratories, Q2 Solutions, Medpace |
| Last Modified Date | 31 January 2026 |
Efficacy Testing Market Overview
Customize Efficacy Testing Market Report market research report
- ✔ Get in-depth analysis of Efficacy Testing market size, growth, and forecasts.
- ✔ Understand Efficacy Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Efficacy Testing
What is the Market Size & CAGR of Efficacy Testing market in 2033?
Efficacy Testing Industry Analysis
Efficacy Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Efficacy Testing Market Analysis Report by Region
Europe Efficacy Testing Market Report:
Europe's efficacy testing market is valued at $1.69 billion in 2023, with projections of reaching $3.32 billion by 2033. The region benefits from advanced healthcare systems and a steady increase in clinical trials across various therapeutic areas.Asia Pacific Efficacy Testing Market Report:
The Asia Pacific region is expected to experience significant growth, from a market size of $0.99 billion in 2023 to $1.94 billion by 2033. This growth is driven by increasing investments in healthcare infrastructure and a growing focus on clinical research, particularly in countries like China and India.North America Efficacy Testing Market Report:
North America holds a leading position in the Efficacy Testing market with a value of $1.81 billion in 2023, anticipated to grow to $3.56 billion by 2033. The robust presence of major pharmaceutical companies and a strong focus on innovation underpin this growth.South America Efficacy Testing Market Report:
The South American market stood at $0.40 billion in 2023 and is projected to reach $0.79 billion by 2033. The improving regulatory landscape and expanding healthcare technology adoption are expected to drive growth in this region.Middle East & Africa Efficacy Testing Market Report:
The Middle East and Africa market is relatively smaller, valued at $0.31 billion in 2023 and expected to grow to $0.61 billion by 2033. Growth in this region is supported by increasing healthcare investments and initiatives aimed at enhancing research capabilities.Tell us your focus area and get a customized research report.
Efficacy Testing Market Analysis By Methodology
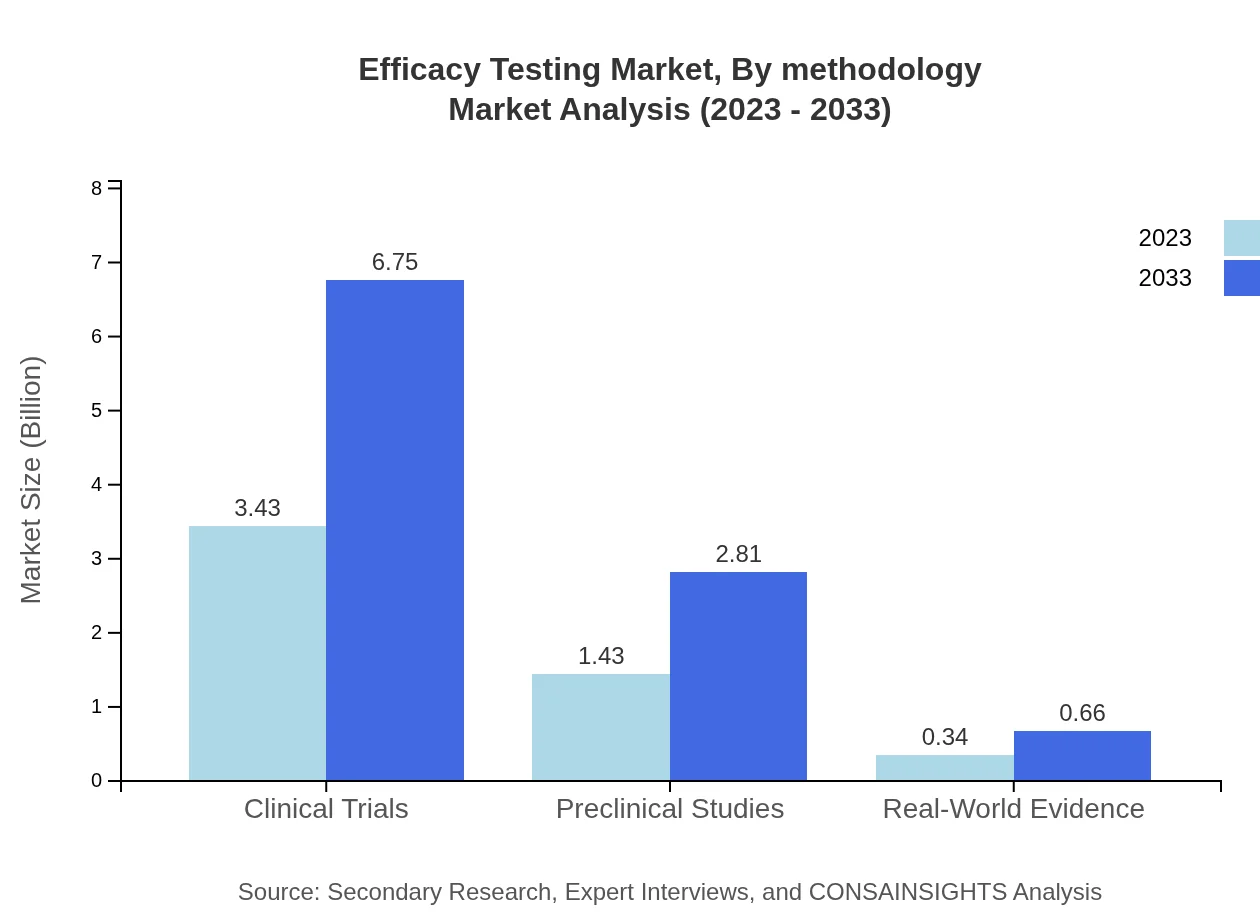
The Efficacy Testing Market by methodology is split between clinical trials and preclinical studies. In 2023, clinical trials hold the largest share at 66%, valued at $3.43 billion, and are expected to rise to $6.75 billion by 2033, continuing to dominate the market due to the high need for human testing of new drugs.
Efficacy Testing Market Analysis By Sector
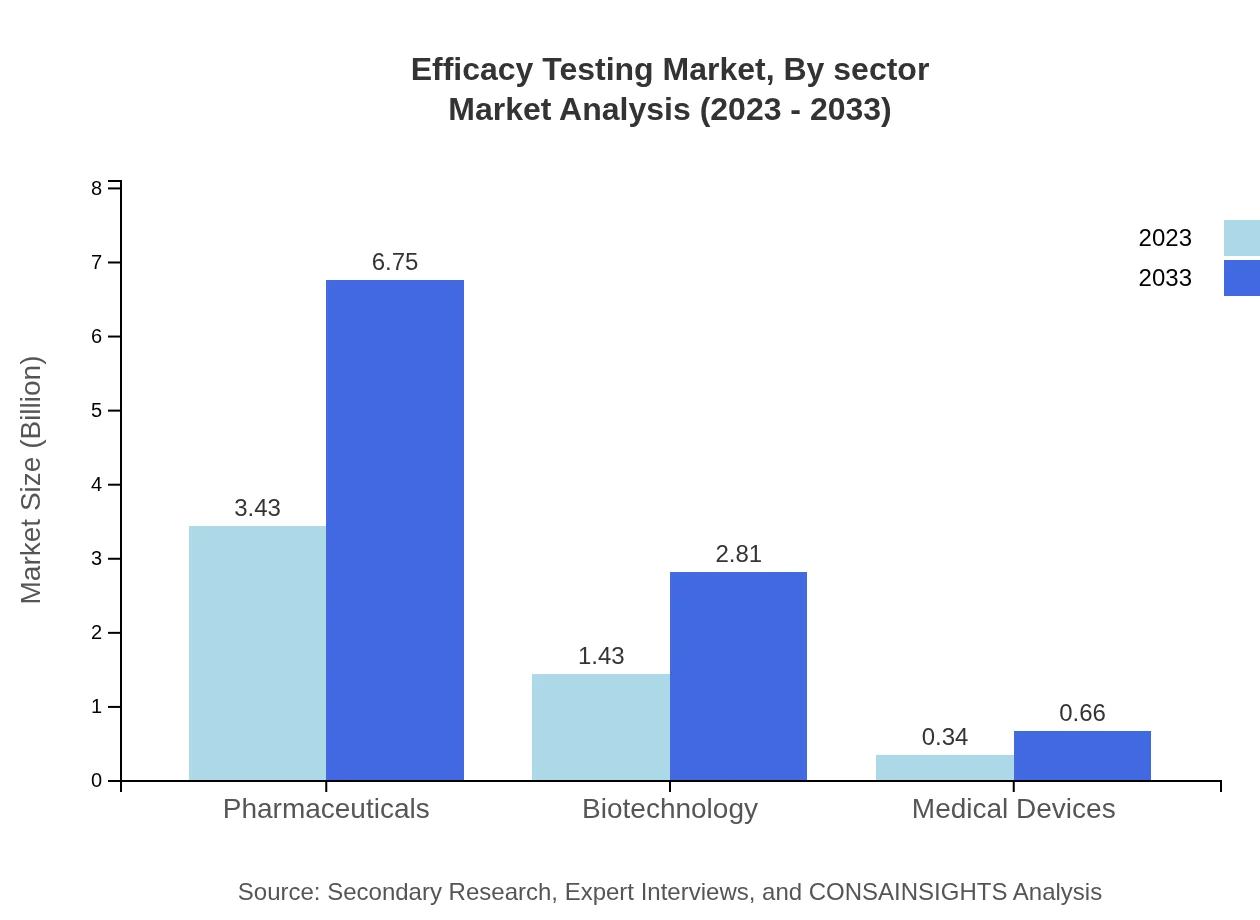
Pharmaceuticals lead the Efficacy Testing Market, valued at $3.43 billion (66%) in 2023, projected to grow to $6.75 billion by 2033. Biotechnology follows, with a size of $1.43 billion (27.52%) in 2023 and expected growth to $2.81 billion. The performance of medical devices, valued at $0.34 billion (6.48%), is also noteworthy as it expands.
Efficacy Testing Market Analysis By Application
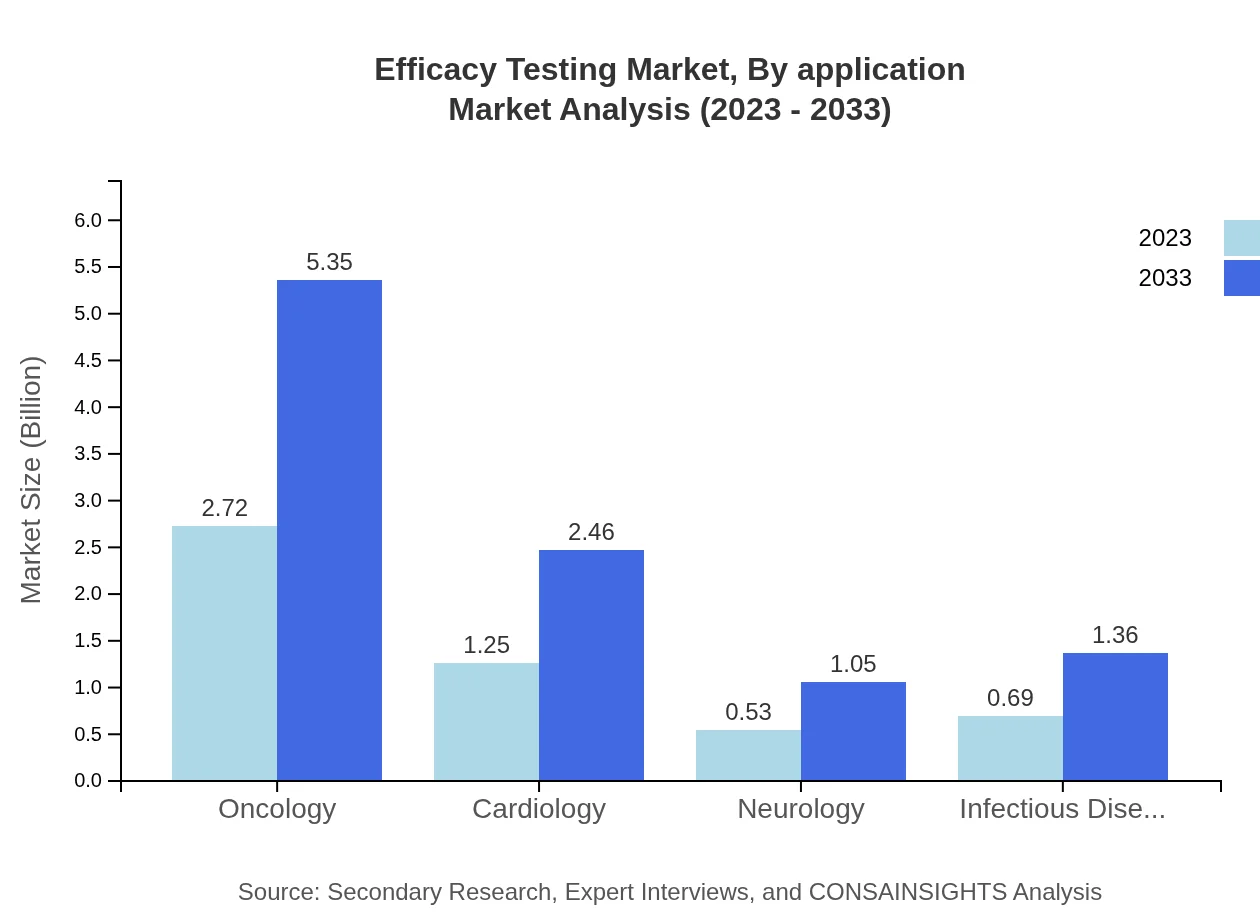
Oncology is the largest application area, accounting for $2.72 billion (52.31%) in 2023, projected to grow to $5.35 billion by 2033. Other important areas include cardiology ($1.25 billion) and neurology ($0.53 billion), both of which are witnessing significant investment in clinical efficacy testing initiatives.
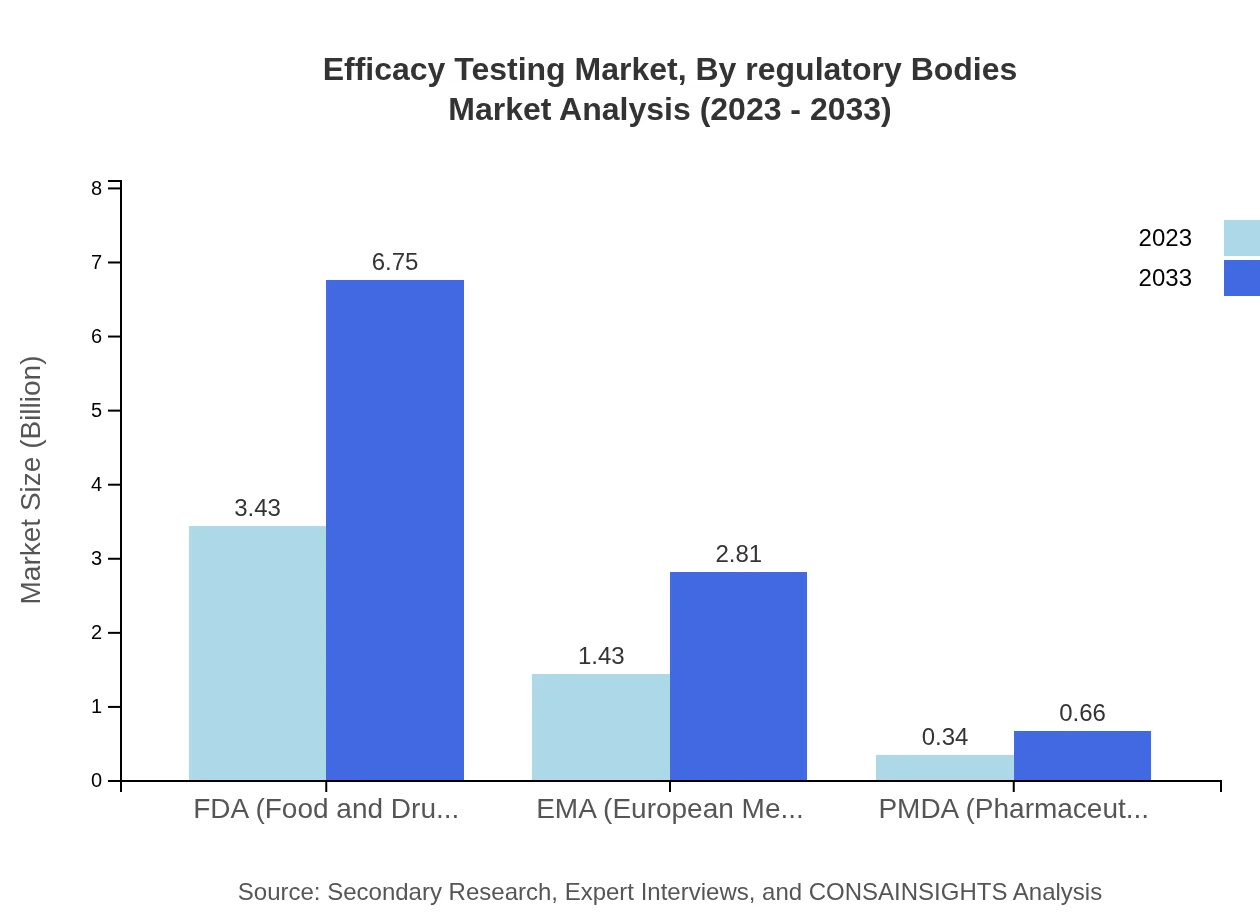
Efficacy Testing Market Analysis By Regulatory Bodies
The Efficacy Testing Market is significantly influenced by regulatory bodies such as the FDA, EMA, and PMDA. The FDA's market share is 66%, with a value of $3.43 billion in 2023, projected to reach $6.75 billion. EMA and PMDA account for 27.52% and 6.48% respectively, reflecting a structured compliance environment that drives efficacy testing standards.
Efficacy Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Efficacy Testing Industry
Covance:
Covance is a global leader in drug development services, offering comprehensive solutions for efficacy testing across pharmaceuticals and biologics.Charles River Laboratories:
Charles River specializes in early-stage preclinical and clinical development services, including efficacy testing, helping clients bring transformative drugs to market.Q2 Solutions:
Q2 Solutions provides a full suite of laboratory services, focusing on enhancing clinical trial performance and ensuring regulatory compliance for efficacy testing.Medpace:
Medpace is a scientifically-driven organization with expertise in various therapeutic areas and offers end-to-end clinical development services, including efficacy testing.We're grateful to work with incredible clients.









FAQs
What is the market size of efficacy Testing?
The efficacy testing market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 6.8% from 2023 to 2033, indicating robust growth driven by innovation in pharmaceuticals and biotechnology sectors.
What are the key market players or companies in this efficacy Testing industry?
Key players in the efficacy testing industry include major pharmaceutical companies, biopharma firms, and clinical research organizations, which are critical in driving advancements in efficacy testing methods and technologies.
What are the primary factors driving the growth in the efficacy testing industry?
Growth in the efficacy testing industry is primarily driven by increased demand for clinical trials, regulatory requirements, and advances in biotechnology and pharmaceutical research, facilitating innovations in drug development.
Which region is the fastest Growing in the efficacy testing?
The fastest-growing region in the efficacy testing market is North America, projected to grow from $1.81 billion in 2023 to $3.56 billion by 2033, followed closely by Europe, Asia Pacific, and Latin America.
Does ConsaInsights provide customized market report data for the efficacy testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the efficacy testing industry, enabling clients to gain deeper insights and data-driven strategies for their business.
What deliverables can I expect from this efficacy testing market research project?
Deliverables include comprehensive market analysis reports, data visualizations, and actionable insights covering trends, market size, competitive analysis, and growth drivers in the efficacy testing sector.
What are the market trends of efficacy testing?
Current market trends in efficacy testing indicate a shift toward personalized medicine, increased reliance on real-world evidence, and digital transformation in clinical trials, enhancing efficiency and patient outcomes.