Electroceuticals Bioelectric Medicine Market Report
Published Date: 31 January 2026 | Report Code: electroceuticals-bioelectric-medicine
Electroceuticals Bioelectric Medicine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Electroceuticals Bioelectric Medicine market, examining market trends, segmentation, regional insights, and forecasts from 2023 to 2033. Key data and insights into the market's dynamics and growth opportunities are highlighted.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
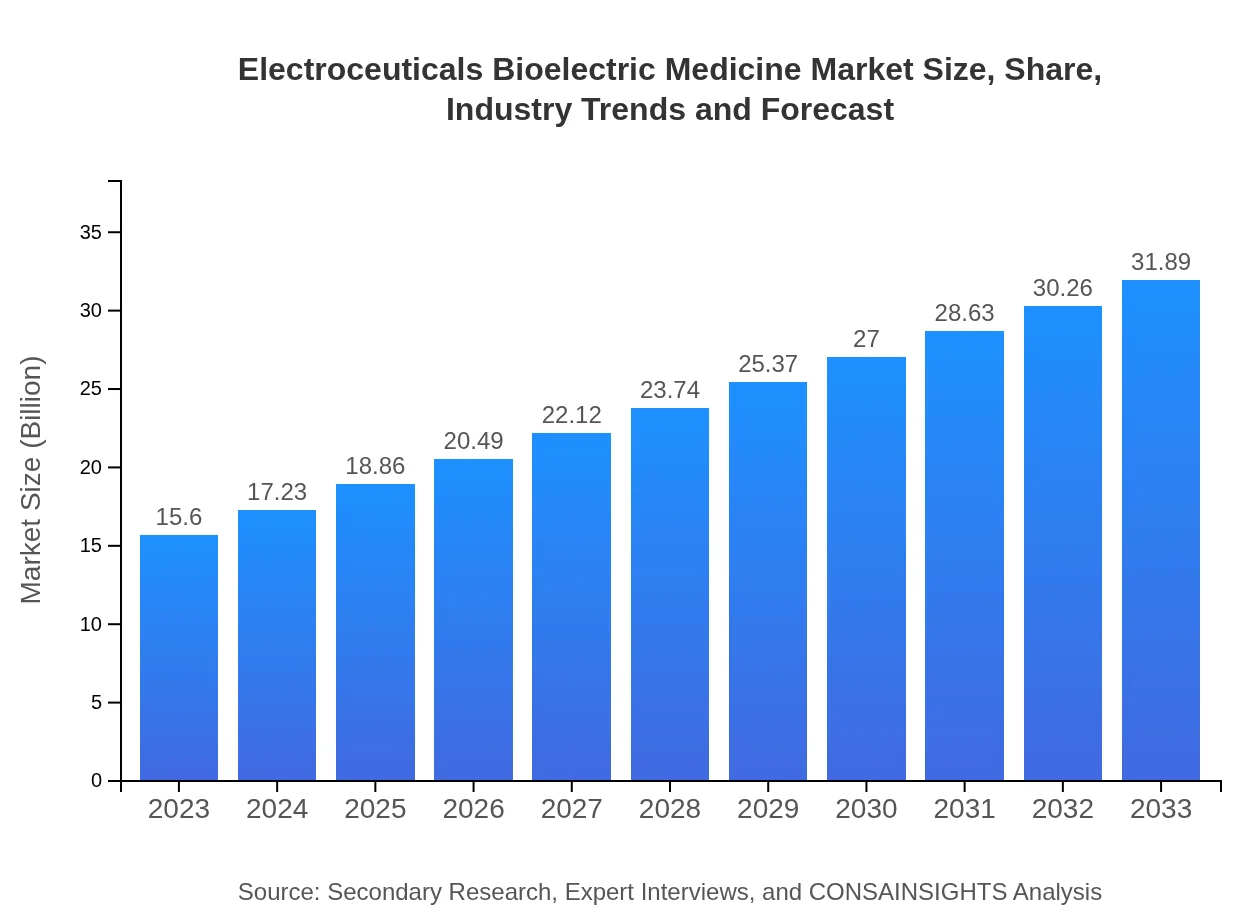
| 2023 Market Size | $15.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $31.89 Billion |
| Top Companies | Medtronic , Boston Scientific, Abbott Laboratories, Nevro Corp |
| Last Modified Date | 31 January 2026 |
Electroceuticals Bioelectric Medicine Market Overview
Customize Electroceuticals Bioelectric Medicine Market Report market research report
- ✔ Get in-depth analysis of Electroceuticals Bioelectric Medicine market size, growth, and forecasts.
- ✔ Understand Electroceuticals Bioelectric Medicine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Electroceuticals Bioelectric Medicine
What is the Market Size & CAGR of Electroceuticals Bioelectric Medicine market in 2023 and 2033?
Electroceuticals Bioelectric Medicine Industry Analysis
Electroceuticals Bioelectric Medicine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Electroceuticals Bioelectric Medicine Market Analysis Report by Region
Europe Electroceuticals Bioelectric Medicine Market Report:
Europe's market is also witnessing growth, projected to increase from $4.08 billion in 2023 to $8.34 billion by 2033. Factors such as the presence of leading market players, increased investment in R&D, and supportive regulatory frameworks significantly contribute to this advancement.Asia Pacific Electroceuticals Bioelectric Medicine Market Report:
The Asia Pacific market is anticipated to witness notable growth, projected to rise from $3.23 billion in 2023 to $6.60 billion in 2033. Factors such as a large population base, increasing healthcare expenditure, and rising prevalence of chronic diseases are fueling market expansion in this region.North America Electroceuticals Bioelectric Medicine Market Report:
North America remains the largest market, expected to expand from $5.70 billion in 2023 to $11.64 billion in 2033. Key drivers include high healthcare expenditure, advanced technological framework, and strong adoption of innovative medical devices in clinical practice.South America Electroceuticals Bioelectric Medicine Market Report:
In South America, the market is expected to grow from $0.85 billion in 2023 to $1.74 billion by 2033. The growth is attributed to rising awareness about bioelectric treatment options and improving healthcare infrastructure across emerging economies.Middle East & Africa Electroceuticals Bioelectric Medicine Market Report:
The Middle East and Africa market is expected to grow from $1.75 billion in 2023 to $3.57 billion by 2033, owing to increased investments in healthcare infrastructure and rising acceptance of advanced medical technologies in the region.Tell us your focus area and get a customized research report.
Electroceuticals Bioelectric Medicine Market Analysis By Product
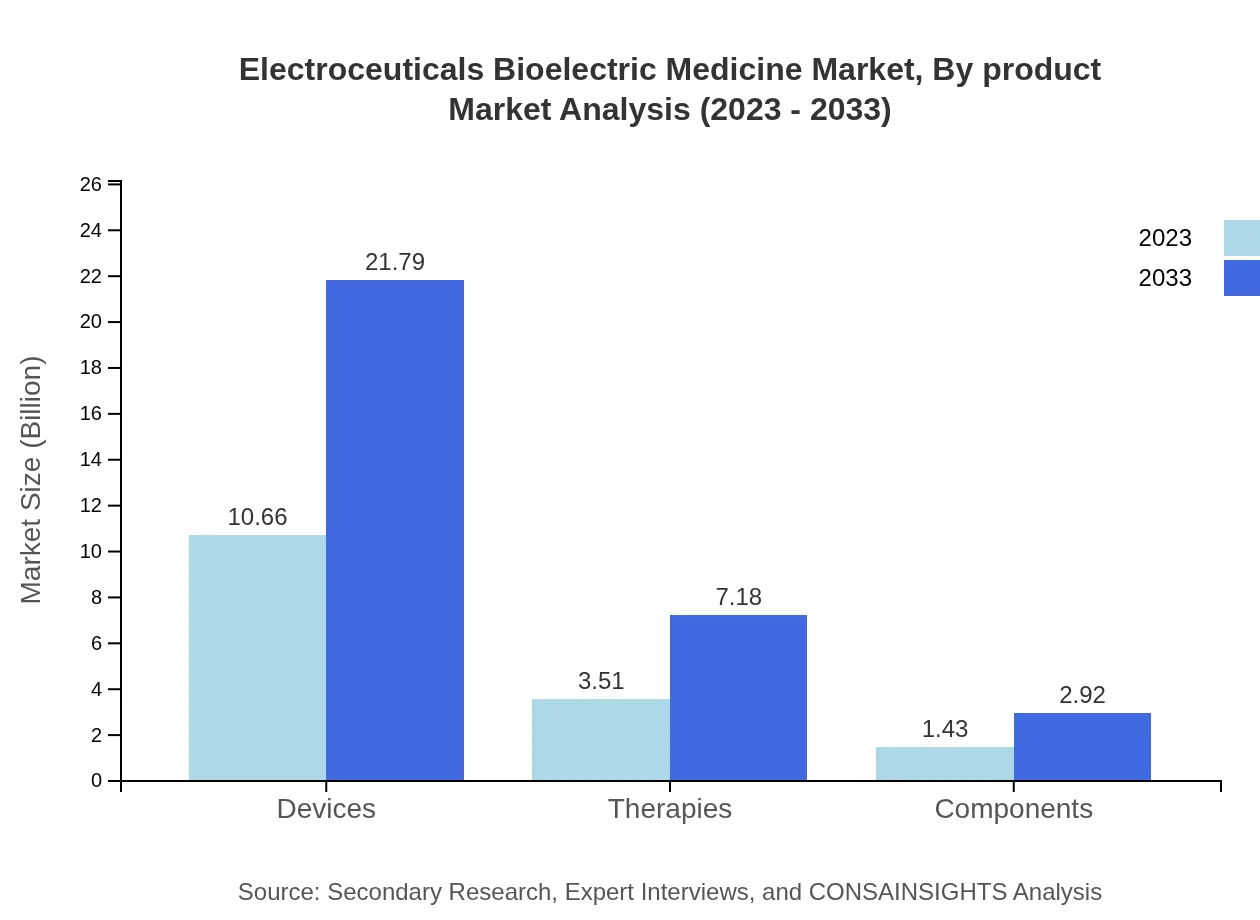
The product segment of the Electroceuticals Bioelectric Medicine market comprises devices, therapies, and components. In 2023, the devices segment is valued at $10.66 billion and is expected to reach $21.79 billion in 2033, maintaining a dominant market share of 68.33%. The therapies segment is anticipated to grow from $3.51 billion to $7.18 billion, with a share of 22.51%, while components will see growth from $1.43 billion to $2.92 billion, holding a 9.16% market share.
Electroceuticals Bioelectric Medicine Market Analysis By Application
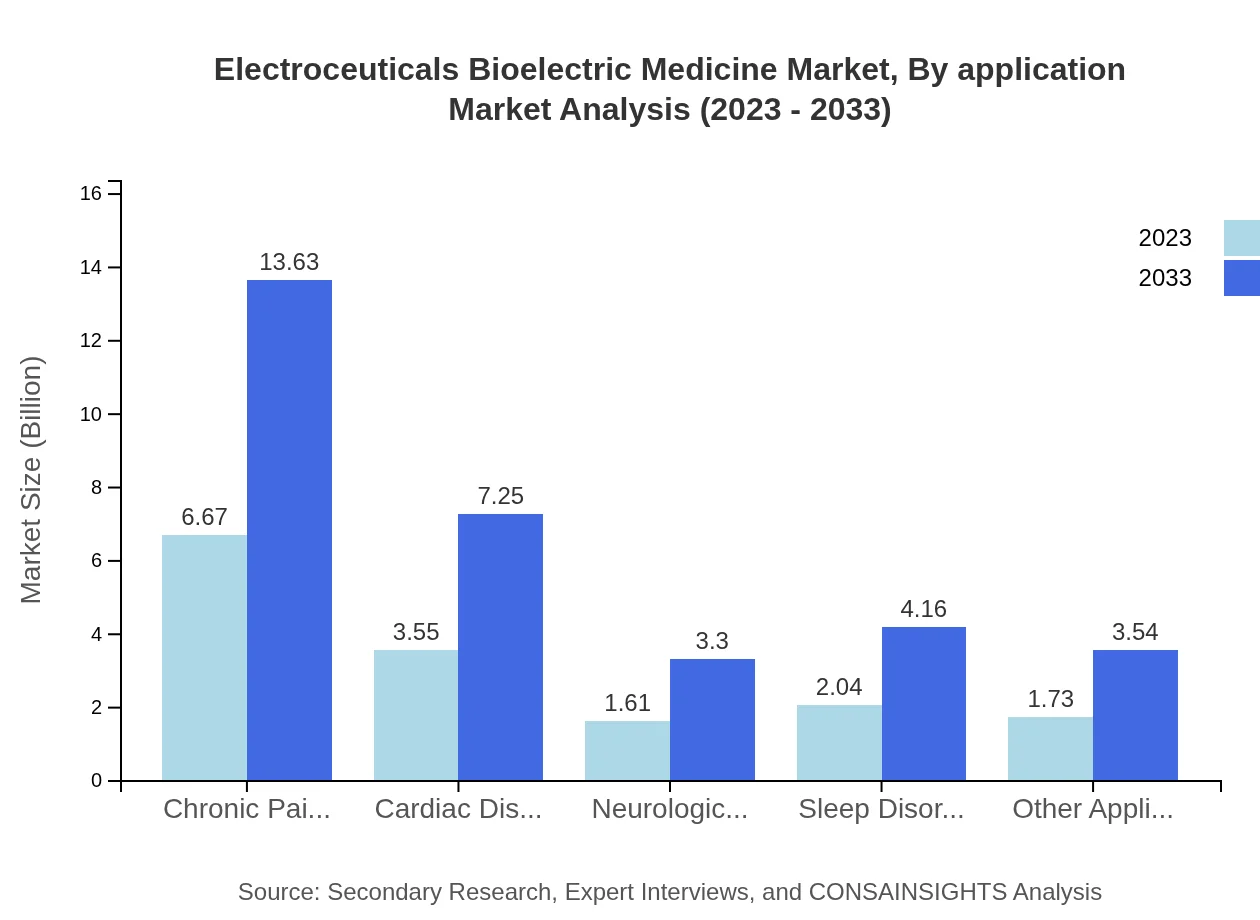
The application segments of the Electroceuticals Bioelectric Medicine market include chronic pain management, cardiac disorders, and neurological disorders. Chronic pain management holds a significant size of $6.67 billion in 2023, projected to reach $13.63 billion by 2033, representing a 42.75% market share. Cardiac disorders and neurological disorders will also show impressive growth trajectories in the same period.
Electroceuticals Bioelectric Medicine Market Analysis By Technology
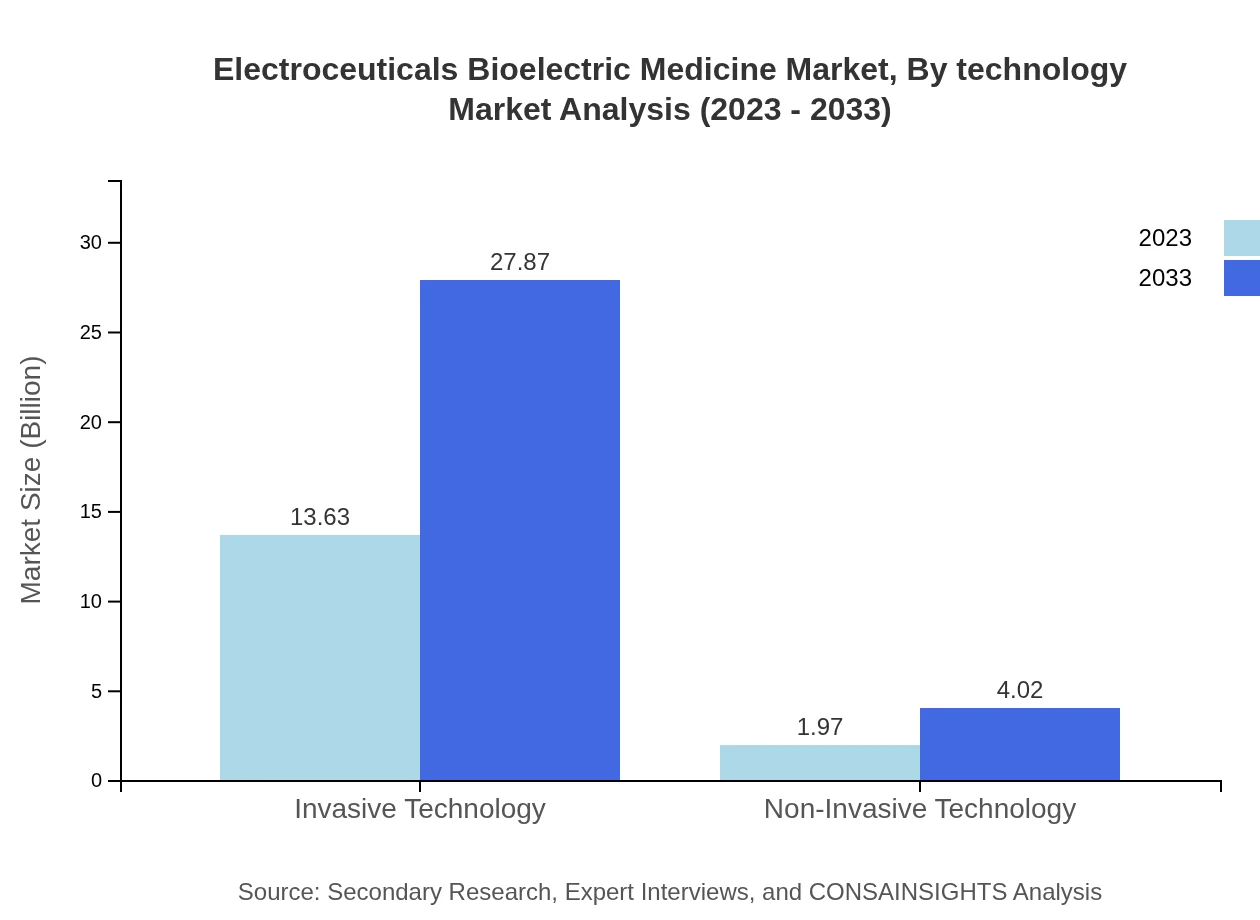
Electroceuticals technology can be categorized into invasive and non-invasive technologies. The invasive technology segment, with a valuation of $13.63 billion in 2023, is projected to reach $27.87 billion by 2033, capturing a market share of 87.39%. In contrast, non-invasive technology is forecasted to grow from $1.97 billion to $4.02 billion, with a share of 12.61%.
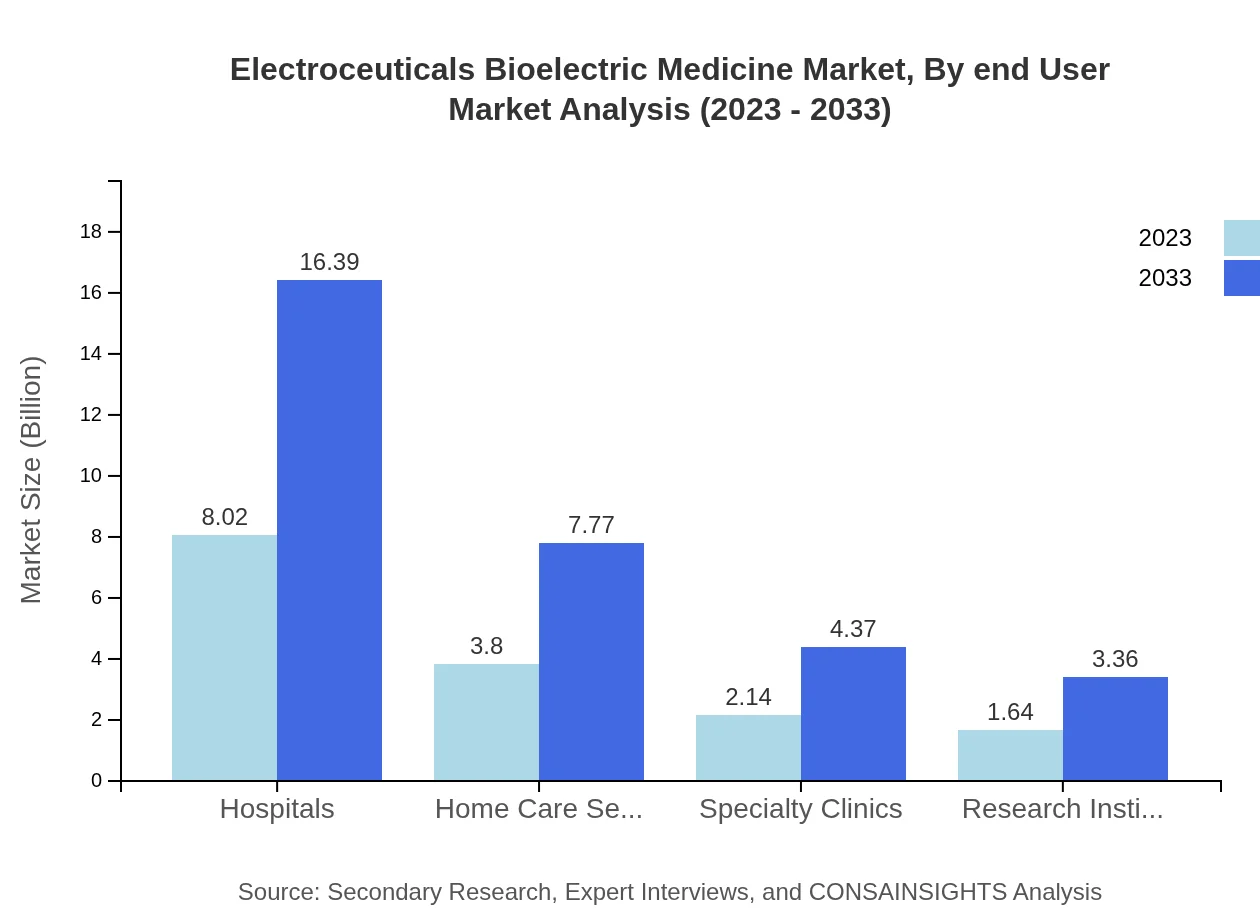
Electroceuticals Bioelectric Medicine Market Analysis By End User
End-users of the Electroceuticals market include hospitals, home care settings, specialty clinics, and research institutes. Hospitals dominate with a size of $8.02 billion in 2023, expected to grow to $16.39 billion by 2033, accounting for a 51.4% market share. Home care settings and research institutes also contribute significantly to the market but with smaller shares.
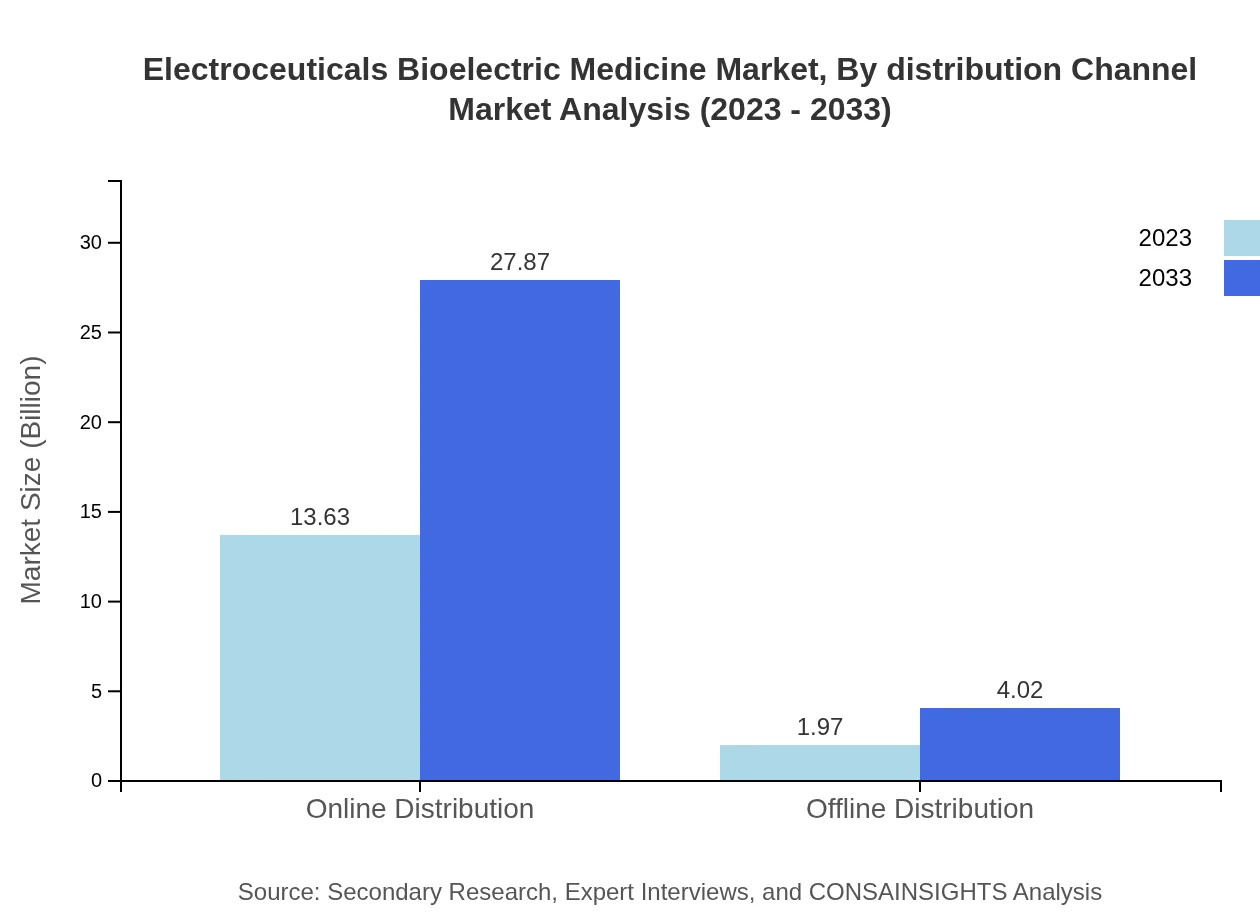
Electroceuticals Bioelectric Medicine Market Analysis By Distribution Channel
Distribution channels for the Electroceuticals Bioelectric Medicine include both online and offline methods. The online distribution channel accounts for a remarkable $13.63 billion in 2023, expected to double to $27.87 billion by 2033, representing an 87.39% share. Offline channels, while smaller, will grow from $1.97 billion to $4.02 billion.
Electroceuticals Bioelectric Medicine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Electroceuticals Bioelectric Medicine Industry
Medtronic :
A key player in the bioelectronic medicine field, Medtronic develops innovative products aimed at treating chronic pain and neurological disorders, leading the way in R&D.Boston Scientific:
Boston Scientific provides technology solutions across various therapeutic areas with a strong focus on cardiac devices and bioelectric stimulation, contributing significantly to patient care improvements.Abbott Laboratories:
Abbott Laboratories is a pioneer in healthcare technologies, developing high-quality electroceutical solutions for heart and neurovascular treatments that enhance patient quality of life.Nevro Corp:
Nevro is renowned for its innovative spinal cord stimulation systems aimed at treating chronic pain, recognized for delivering advanced technology and patient-focused solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of electroceuticals Bioelectric Medicine?
The electroceuticals-bioelectric medicine market is currently valued at approximately $15.6 billion and is projected to grow at a CAGR of 7.2% from 2023 to 2033.
What are the key market players or companies in this electroceuticals Bioelectric Medicine industry?
Key players in the electroceuticals-bioelectric medicine market include leading medical device manufacturers such as Medtronic, Boston Scientific, and Abbott Laboratories, which dominate the landscape with innovative product offerings and expansive market reach.
What are the primary factors driving the growth in the electroceuticals Bioelectric Medicine industry?
Growth in the electroceuticals-bioelectric medicine industry is primarily driven by increasing prevalence of chronic diseases, rising healthcare expenditures, advancements in bioelectric technology, and growing consumer awareness of non-invasive treatment options.
Which region is the fastest Growing in the electroceuticals Bioelectric Medicine?
The Asia Pacific region is the fastest-growing market for electroceuticals-bioelectric medicine, expected to expand from $3.23 billion in 2023 to $6.60 billion by 2033, benefiting from rising healthcare investments and expanding geriatric populations.
Does ConsaInsights provide customized market report data for the electroceuticals Bioelectric Medicine industry?
Yes, ConsaInsights offers customized market report data tailored specifically for clients in the electroceuticals-bioelectric medicine industry, enabling organizations to align insights with their strategic objectives.
What deliverables can I expect from this electroceuticals Bioelectric Medicine market research project?
Deliverables from the electroceuticals-bioelectric medicine market research project include comprehensive market analysis reports, strategic recommendations, competitive landscape assessments, and detailed insights into regional and segment performance.
What are the market trends of electroceuticals Bioelectric Medicine?
Key trends in the electroceuticals-bioelectric medicine market include a shift towards non-invasive therapies, increasing integration of AI in device functionality, growing adoption of Internet of Things (IoT) for remote patient monitoring, and heightened focus on personalized medicine.