Electronic Clinical Outcome Assessment Solutions Ecoa Market Report
Published Date: 31 January 2026 | Report Code: electronic-clinical-outcome-assessment-solutions-ecoa
Electronic Clinical Outcome Assessment Solutions Ecoa Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive market report on Electronic Clinical Outcome Assessment Solutions (ECOA) covers critical insights and trends over the forecast period from 2023 to 2033, providing an assessment of the market size, growth rates, segmentation, and key players in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
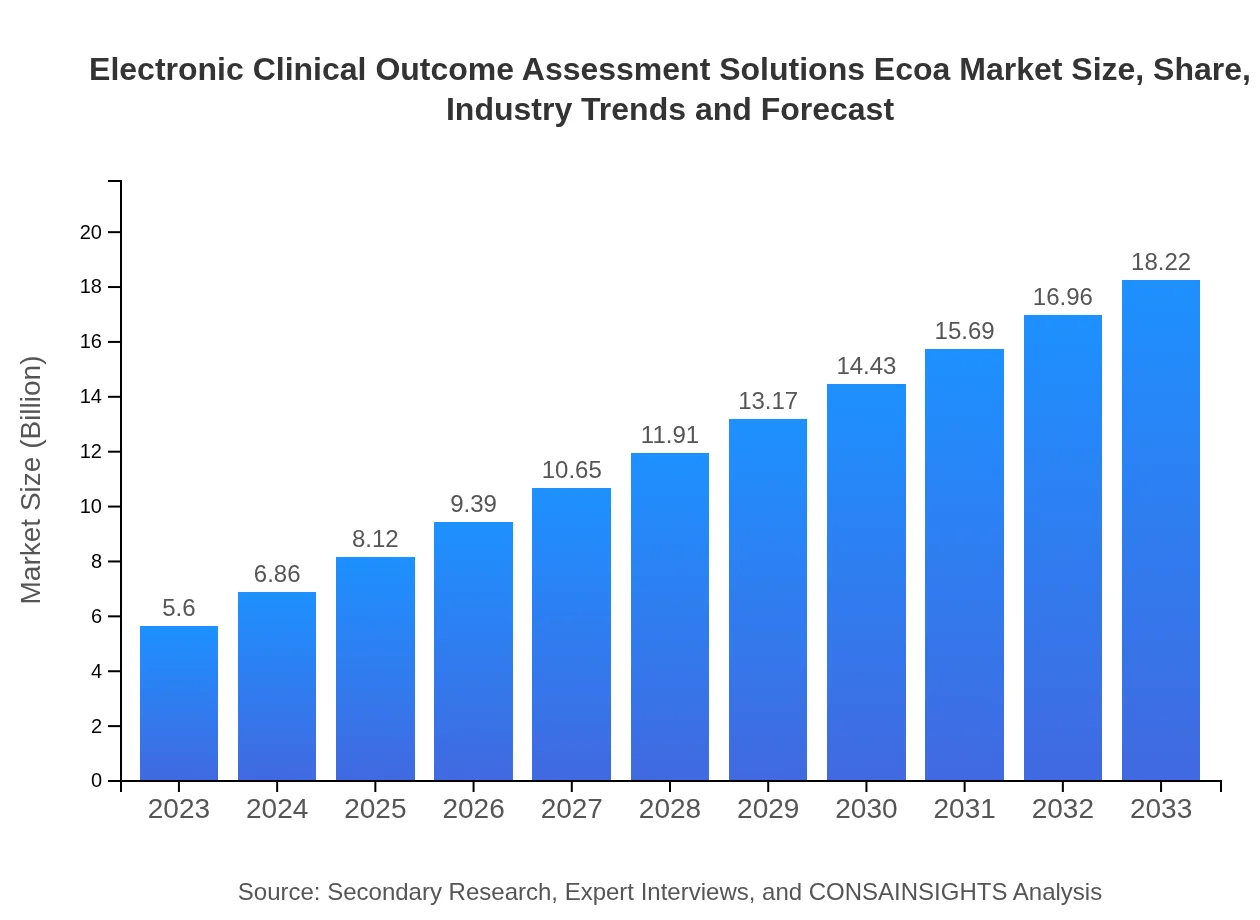
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 12% |
| 2033 Market Size | $18.22 Billion |
| Top Companies | Medidata Solutions, Oracle, eClinical Solutions, Parexel International |
| Last Modified Date | 31 January 2026 |
Electronic Clinical Outcome Assessment Solutions Ecoa Market Overview
Customize Electronic Clinical Outcome Assessment Solutions Ecoa Market Report market research report
- ✔ Get in-depth analysis of Electronic Clinical Outcome Assessment Solutions Ecoa market size, growth, and forecasts.
- ✔ Understand Electronic Clinical Outcome Assessment Solutions Ecoa's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Electronic Clinical Outcome Assessment Solutions Ecoa
What is the Market Size & CAGR of Electronic Clinical Outcome Assessment Solutions Ecoa market in 2023?
Electronic Clinical Outcome Assessment Solutions Ecoa Industry Analysis
Electronic Clinical Outcome Assessment Solutions Ecoa Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Electronic Clinical Outcome Assessment Solutions Ecoa Market Analysis Report by Region
Europe Electronic Clinical Outcome Assessment Solutions Ecoa Market Report:
Europe's ECOA market growth is notable, expanding from $1.58 billion in 2023 to $5.13 billion by 2033, driven by regulatory support and a focus on patient-centric approaches in clinical trial processes.Asia Pacific Electronic Clinical Outcome Assessment Solutions Ecoa Market Report:
The Asia Pacific region is set to witness substantial growth, with market size projected to grow from $1.04 billion in 2023 to $3.39 billion by 2033, fueled by increasing adoption of digital health solutions and the rising number of clinical trials.North America Electronic Clinical Outcome Assessment Solutions Ecoa Market Report:
North America dominates the ECOA market with an estimated size of $2.17 billion in 2023, expected to grow to $7.07 billion by 2033, supported by advanced healthcare systems and high technology adoption rates.South America Electronic Clinical Outcome Assessment Solutions Ecoa Market Report:
The Latin American market, albeit smaller in scale, shows potential growth from $0.18 billion in 2023 to $0.58 billion in 2033, driven by better healthcare infrastructure and rising investments in clinical research.Middle East & Africa Electronic Clinical Outcome Assessment Solutions Ecoa Market Report:
In the Middle East and Africa, the market is anticipated to grow from $0.63 billion in 2023 to $2.04 billion by 2033, influenced by increased investments in healthcare technology and modernization efforts.Tell us your focus area and get a customized research report.
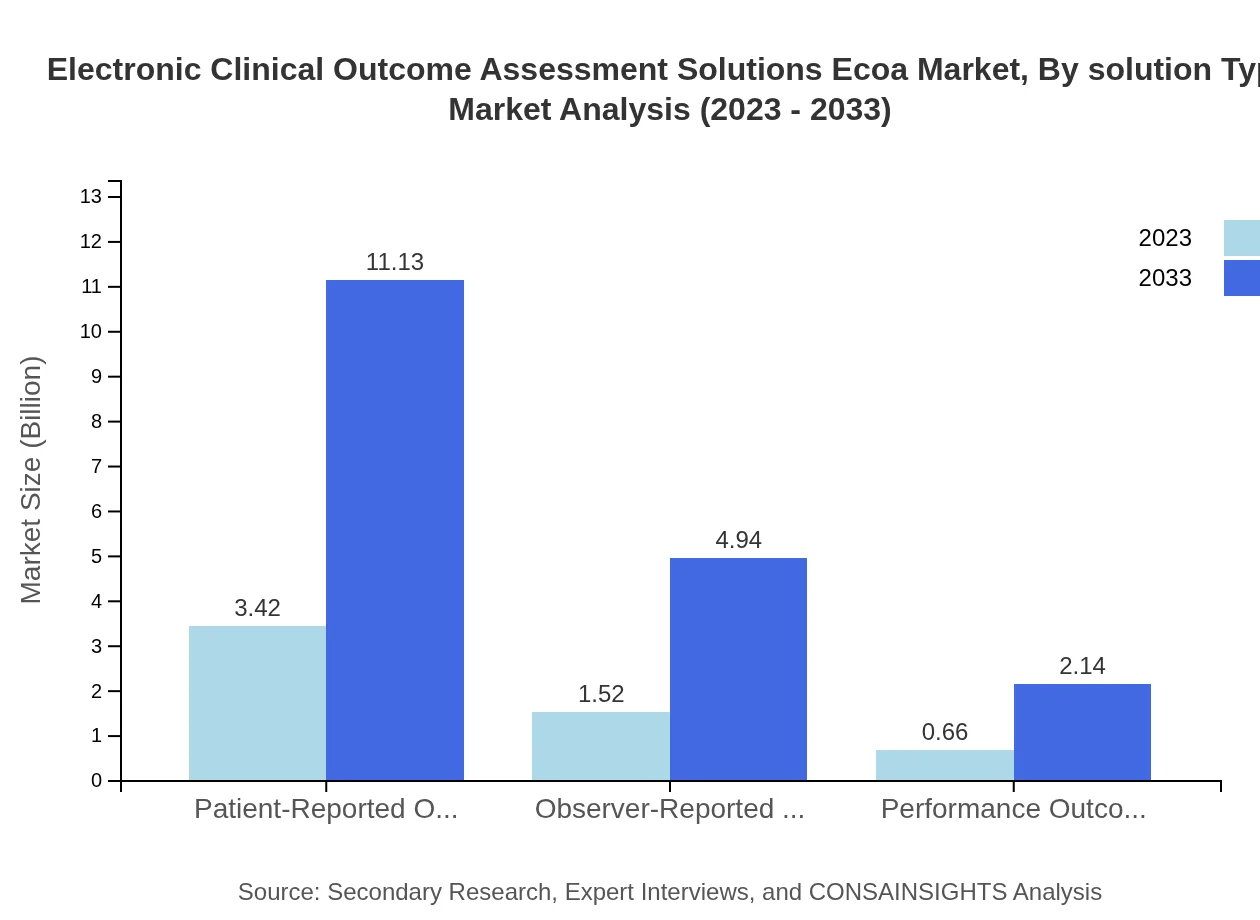
Electronic Clinical Outcome Assessment Solutions Ecoa Market Analysis By Solution Type
In the solution type segment, the patient-reported outcomes lead, valued at $3.42 billion in 2023 and expected to reach $11.13 billion by 2033, holding a 61.1% market share. Other essential segments include observer-reported outcomes, market size expected to grow from $1.52 billion to $4.94 billion, and performance outcomes, projected to rise from $0.66 billion to $2.14 billion.
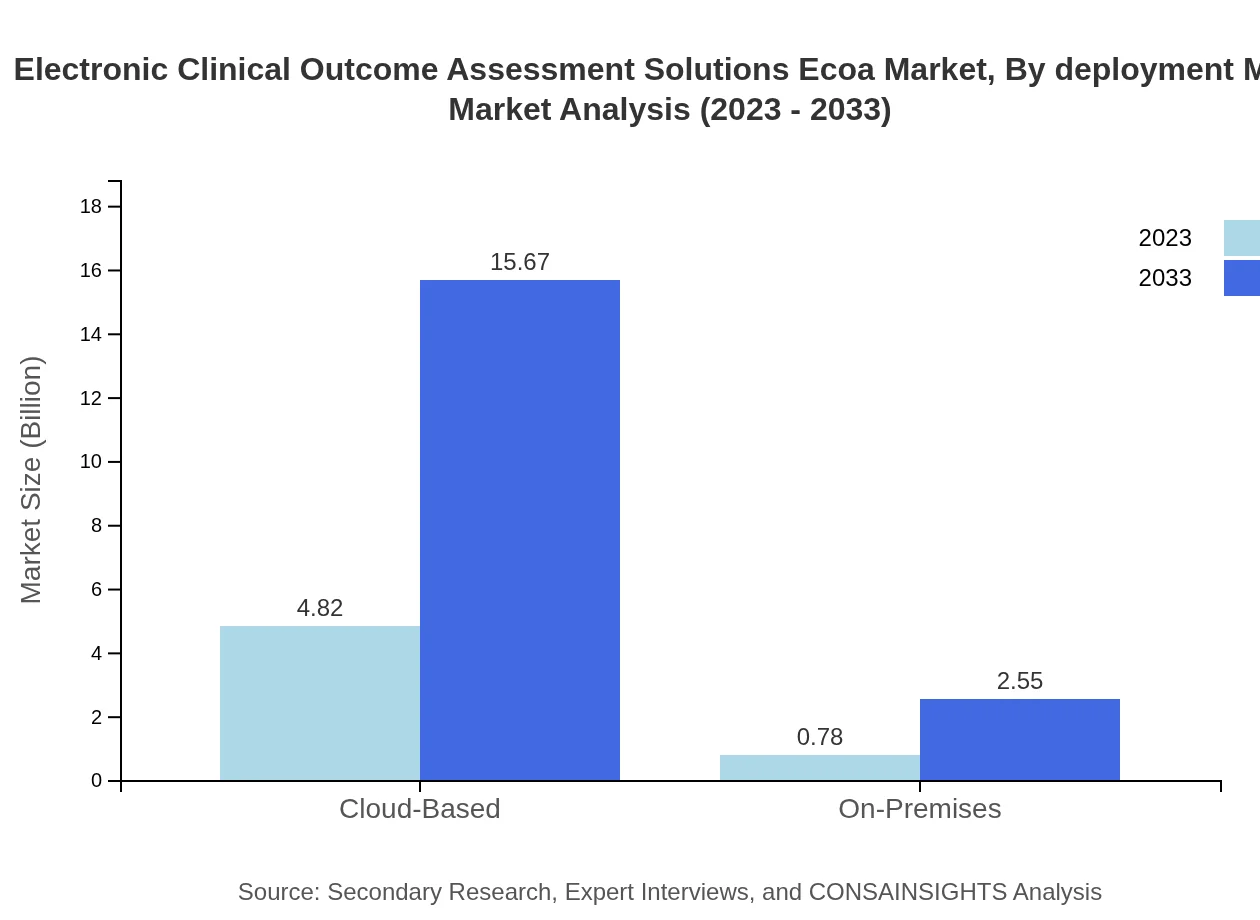
Electronic Clinical Outcome Assessment Solutions Ecoa Market Analysis By Deployment Mode
The cloud-based deployment mode dominates the market with a substantial size of $4.82 billion in 2023, projected to rise to $15.67 billion in 2033, representing 86% share due to its efficiency and cost-effectiveness. In contrast, on-premises solutions, valued at $0.78 billion, offer more control and security for clients, expected to reach $2.55 billion by 2033.
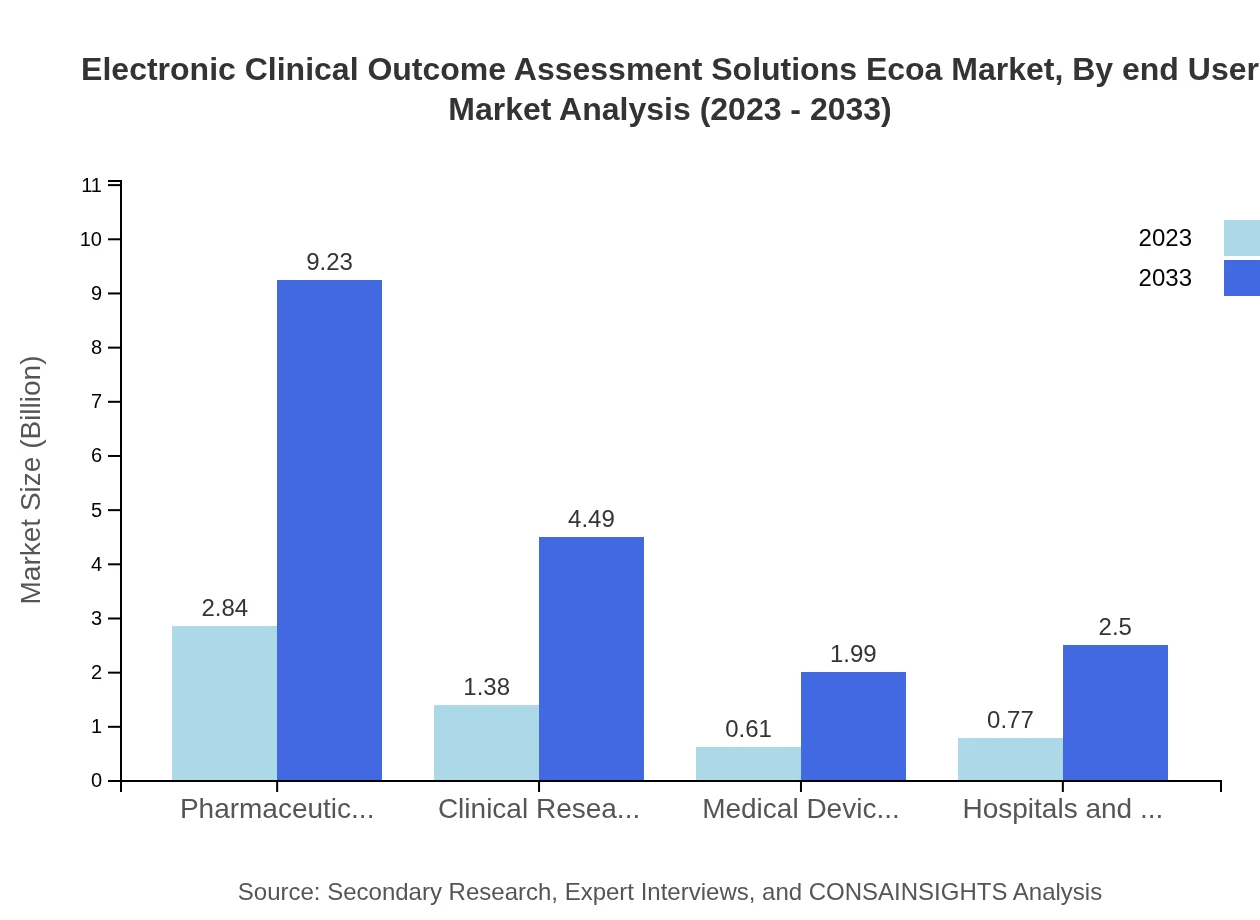
Electronic Clinical Outcome Assessment Solutions Ecoa Market Analysis By End User
Pharmaceutical companies represent the largest share of the ECOA market, accounting for $2.84 billion in 2023 and expected to expand to $9.23 billion by 2033, comprising approximately 50.66% market share. This is followed by clinical research organizations, hospitals, and clinics, further emphasizing collaboration in drug development and patient care.
Electronic Clinical Outcome Assessment Solutions Ecoa Market Analysis By Region
Geographically, the North American region leads the market, followed by Europe and Asia Pacific. Emerging markets in Latin America and the Middle East and Africa exhibit rapid growth potential, suggesting a diversification of opportunities for technology providers.
Electronic Clinical Outcome Assessment Solutions Ecoa Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Electronic Clinical Outcome Assessment Solutions Ecoa Industry
Medidata Solutions:
A leader in cloud-based solutions for clinical trials, Medidata offers innovative ECOA tools enabling streamlined data collection and improved patient engagement.Oracle:
Oracle's comprehensive clinical trial management solutions incorporate ECOA capabilities, facilitating efficient data capture and regulatory compliance.eClinical Solutions:
This company specialized in cloud-based solutions that enhance clinical data management, with a strong emphasis on ECOA to support global clinical trials.Parexel International:
A prominent CRO that integrates ECOA solutions into its clinical services, providing comprehensive data management support in trials.We're grateful to work with incredible clients.









FAQs
What is the market size of electronic clinical outcome assessment solutions (eCOA)?
The electronic Clinical Outcome Assessment (eCOA) market is currently valued at approximately $5.6 billion in 2023, with a expected growth rate (CAGR) of 12% projected through 2033, indicating robust demand and expansion.
What are the key market players or companies in the eCOA industry?
Key market players in the eCOA industry include pharmaceutical companies, clinical research organizations (CROs), medical device manufacturers, as well as hospitals and clinics that utilize eCOA solutions for enhancing patient care and data accuracy.
What are the primary factors driving the growth in the eCOA industry?
The growth of the eCOA market is primarily fueled by an increasing focus on patient-centric approaches, technological advancements in data capture, regulatory compliance, and the rising need for more effective clinical trials.
Which region is the fastest Growing in the eCOA industry?
North America is the fastest-growing region in the eCOA market, with its market size expected to grow from $2.17 billion in 2023 to $7.07 billion by 2033, bolstered by high healthcare expenditure and technological adoption.
Does ConsaInsights provide customized market report data for the eCOA industry?
Yes, Consainsights offers customized market report data tailored to specific needs in the eCOA industry, allowing clients to obtain insights relevant to their unique market segments or geographical areas.
What deliverables can I expect from this eCOA market research project?
Deliverables from the eCOA market research project typically include detailed market analyses, growth forecasts, competitive landscape evaluations, and segment-specific insights, providing comprehensive information for strategic decision-making.
What are the market trends of eCOA?
Key trends in the eCOA market include the shift towards cloud-based solutions, increased emphasis on real-time data collection, integration of patient-reported outcomes, and the growing demand for mobile health applications.