Electronic Trial Master File Etmf Market Report
Published Date: 31 January 2026 | Report Code: electronic-trial-master-file-etmf
Electronic Trial Master File Etmf Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Electronic Trial Master File (eTMF) market, focusing on current trends, regional insights, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
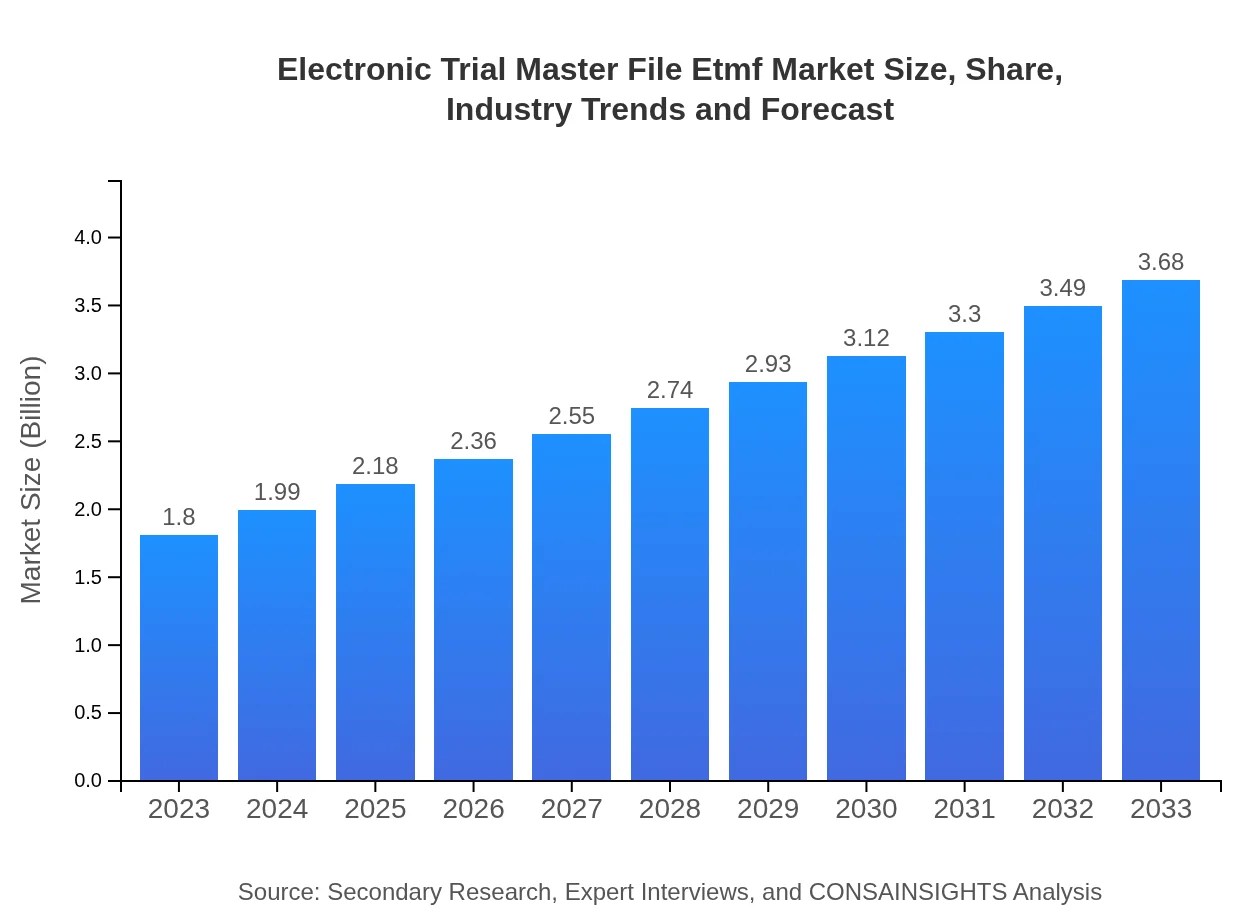
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $3.68 Billion |
| Top Companies | Veeva Systems, Medidata Solutions, Oracle Corporation, TransCelerate BioPharma, MasterControl |
| Last Modified Date | 31 January 2026 |
Electronic Trial Master File Etmf Market Overview
Customize Electronic Trial Master File Etmf Market Report market research report
- ✔ Get in-depth analysis of Electronic Trial Master File Etmf market size, growth, and forecasts.
- ✔ Understand Electronic Trial Master File Etmf's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Electronic Trial Master File Etmf
What is the Market Size & CAGR of Electronic Trial Master File Etmf market in 2023?
Electronic Trial Master File Etmf Industry Analysis
Electronic Trial Master File Etmf Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Electronic Trial Master File Etmf Market Analysis Report by Region
Europe Electronic Trial Master File Etmf Market Report:
Europe’s market, valued at $0.60 billion in 2023 and expected to reach $1.23 billion by 2033, benefits from a high number of clinical trials and strong regulatory compliance culture. Countries like Germany, the UK, and France are significant contributors to the region's market growth.Asia Pacific Electronic Trial Master File Etmf Market Report:
In 2023, the Asia Pacific eTMF market is valued at $0.33 billion, projected to grow to $0.66 billion by 2033. Growth is attributed to increasing clinical trials in emerging economies and the demand for advanced electronic solutions. Countries like India and China are at the forefront of this growth due to enhanced regulatory frameworks and growing investments in clinical research.North America Electronic Trial Master File Etmf Market Report:
North America leads the eTMF market with a size of $0.62 billion in 2023, surging to $1.26 billion by 2033. The growth is primarily driven by technological advancements, a robust pharmaceutical sector, and stringent regulatory compliance needs pushing companies toward eTMF solutions.South America Electronic Trial Master File Etmf Market Report:
The South American market, valued at $0.05 billion in 2023 and projected to reach $0.11 billion by 2033, displays slow but steady growth. The low adoption rate of digital solutions poses challenges, yet increasing regulatory requirements are driving demand for more organized trial documentation.Middle East & Africa Electronic Trial Master File Etmf Market Report:
The Middle East and Africa eTMF market is anticipated to grow from $0.20 billion in 2023 to $0.41 billion by 2033. Although it represents a smaller market share, increasing focus on enhancing clinical trial efficiency and regulatory compliance is driving adoption in this region.Tell us your focus area and get a customized research report.
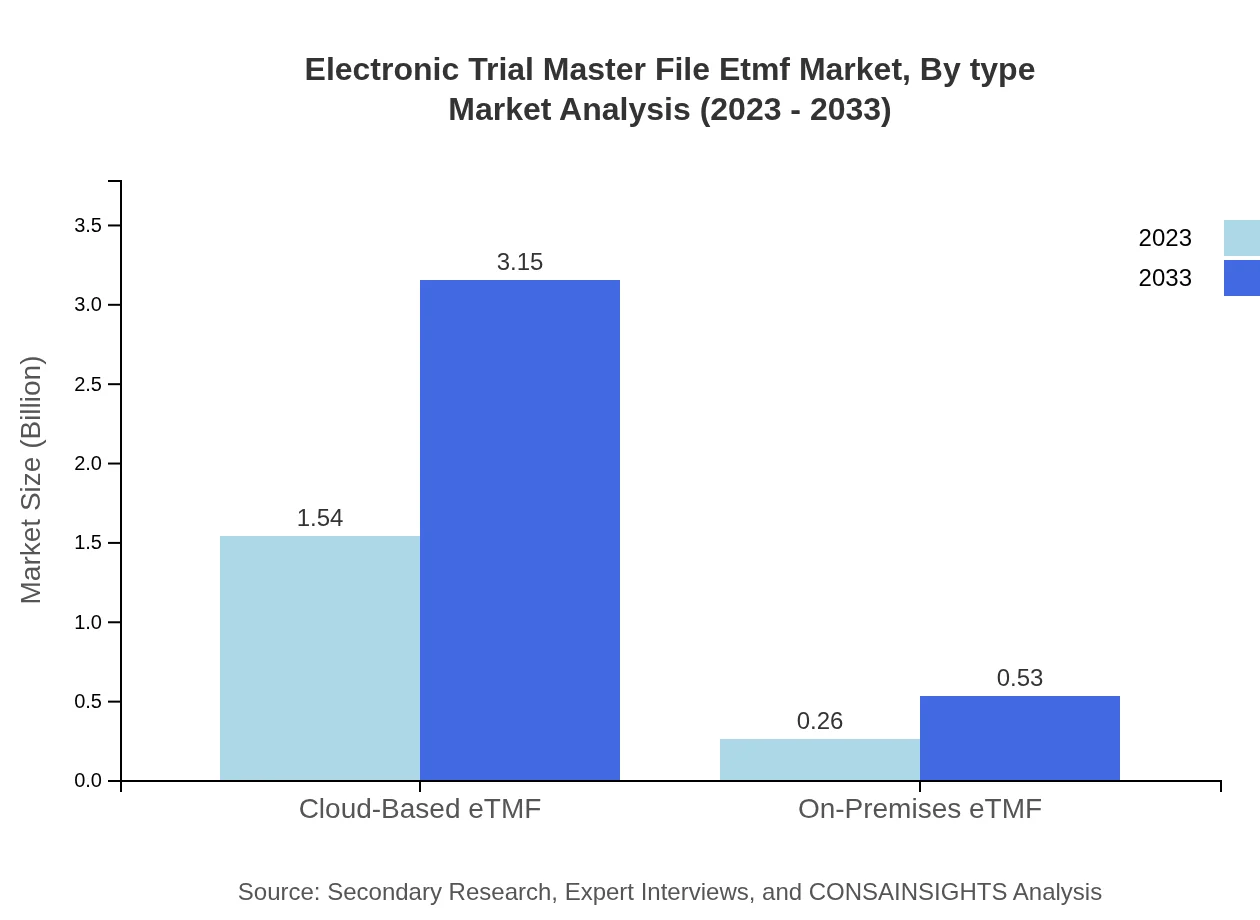
Electronic Trial Master File Etmf Market Analysis By Type
The market is predominantly driven by cloud-based eTMF solutions, which are expected to grow from $1.54 billion in 2023 to $3.15 billion by 2033, accounting for approximately 85.58% of the market share. In contrast, on-premises eTMF solutions are projected to grow from $0.26 billion to $0.53 billion, maintaining 14.42% market share.
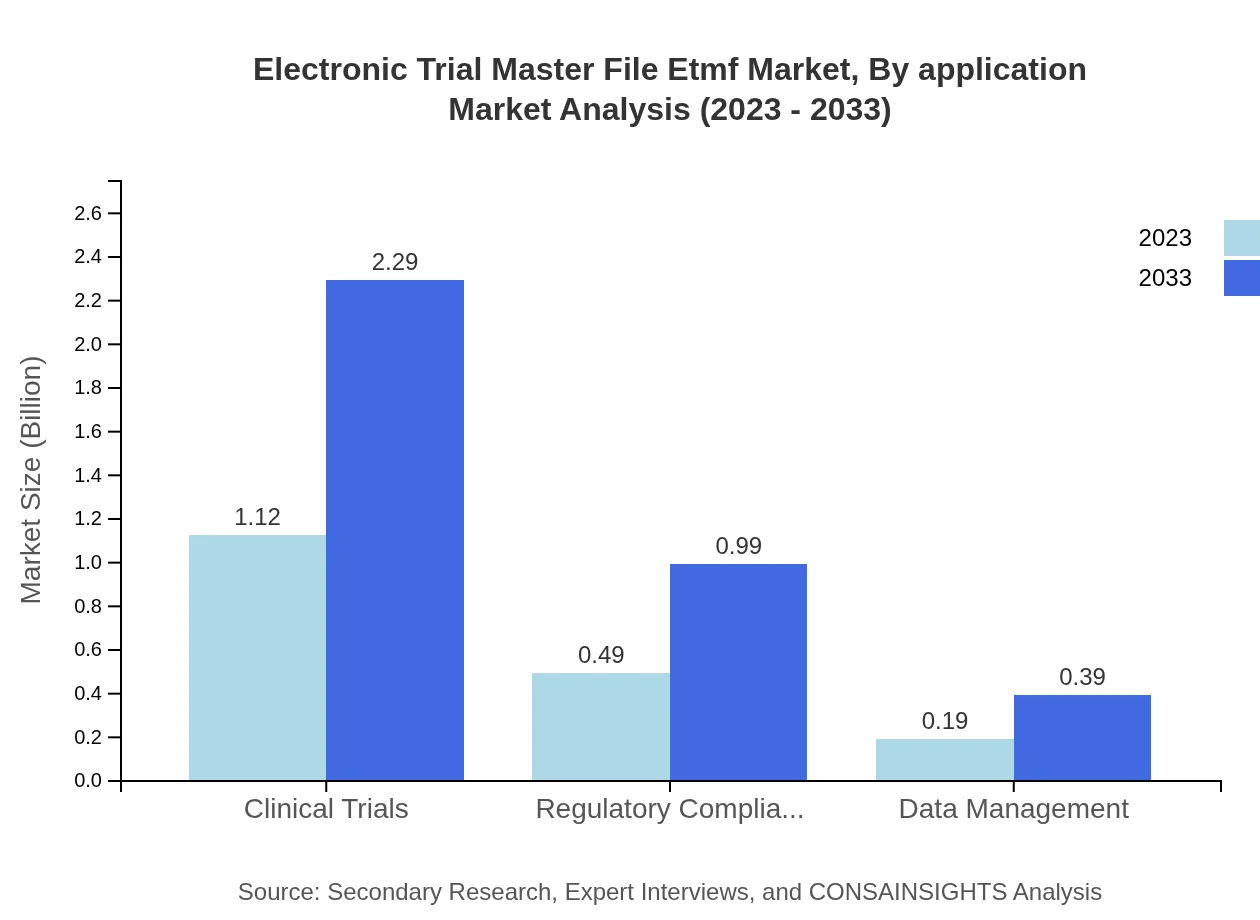
Electronic Trial Master File Etmf Market Analysis By Application
Clinical trials represent a significant portion of the eTMF market, with a valuation of $1.12 billion in 2023, expected to rise to $2.29 billion by 2033. Regulatory compliance solutions also play a crucial role, with a similar growth trajectory, highlighting the industry's need to meet stringent regulations.
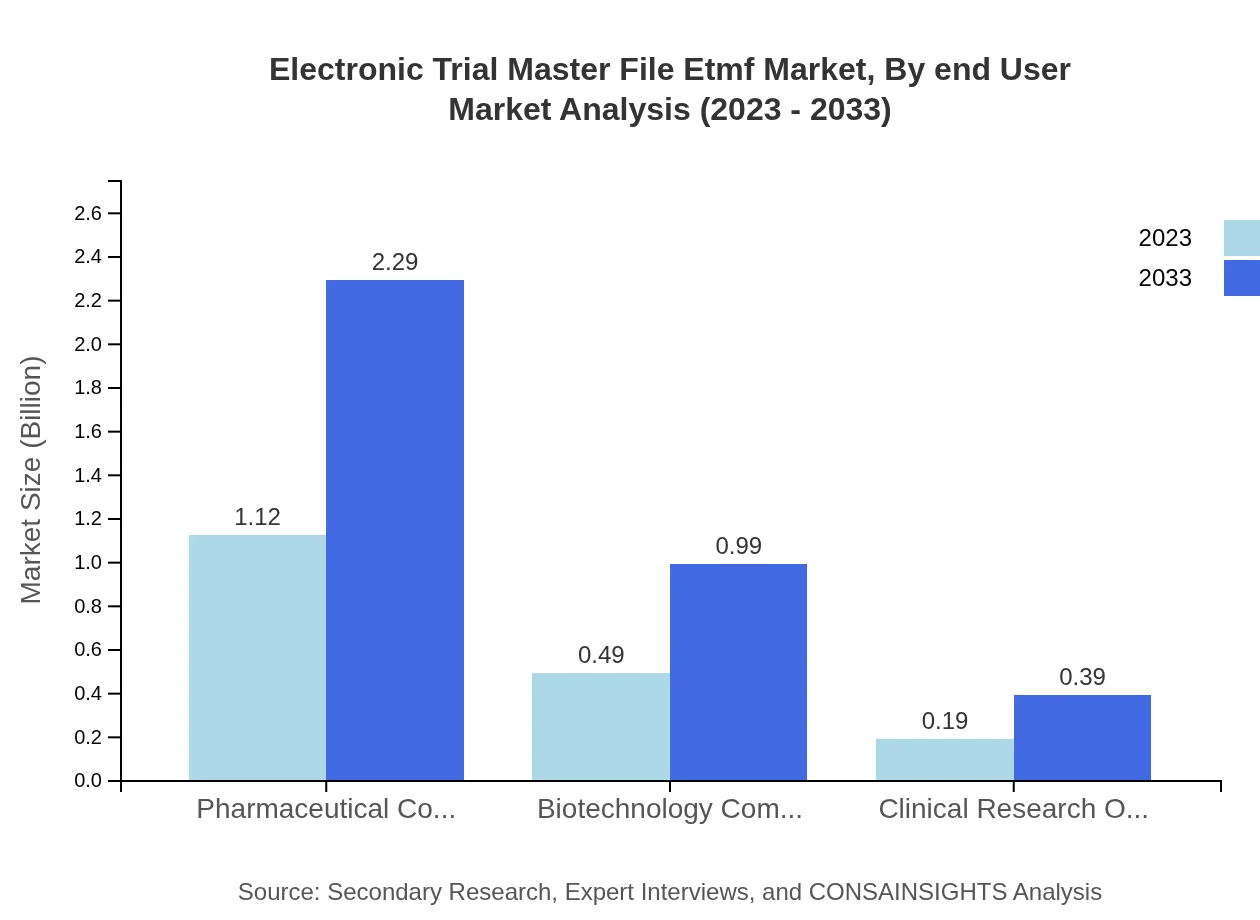
Electronic Trial Master File Etmf Market Analysis By End User
Pharmaceutical companies hold the largest share, valued at $1.12 billion in 2023 and set to grow to $2.29 billion, reflecting the industry's reliance on effective eTMF solutions. Biotechnology companies also represent a significant segment, with a market size of $0.49 billion rising to $0.99 billion during the same period.
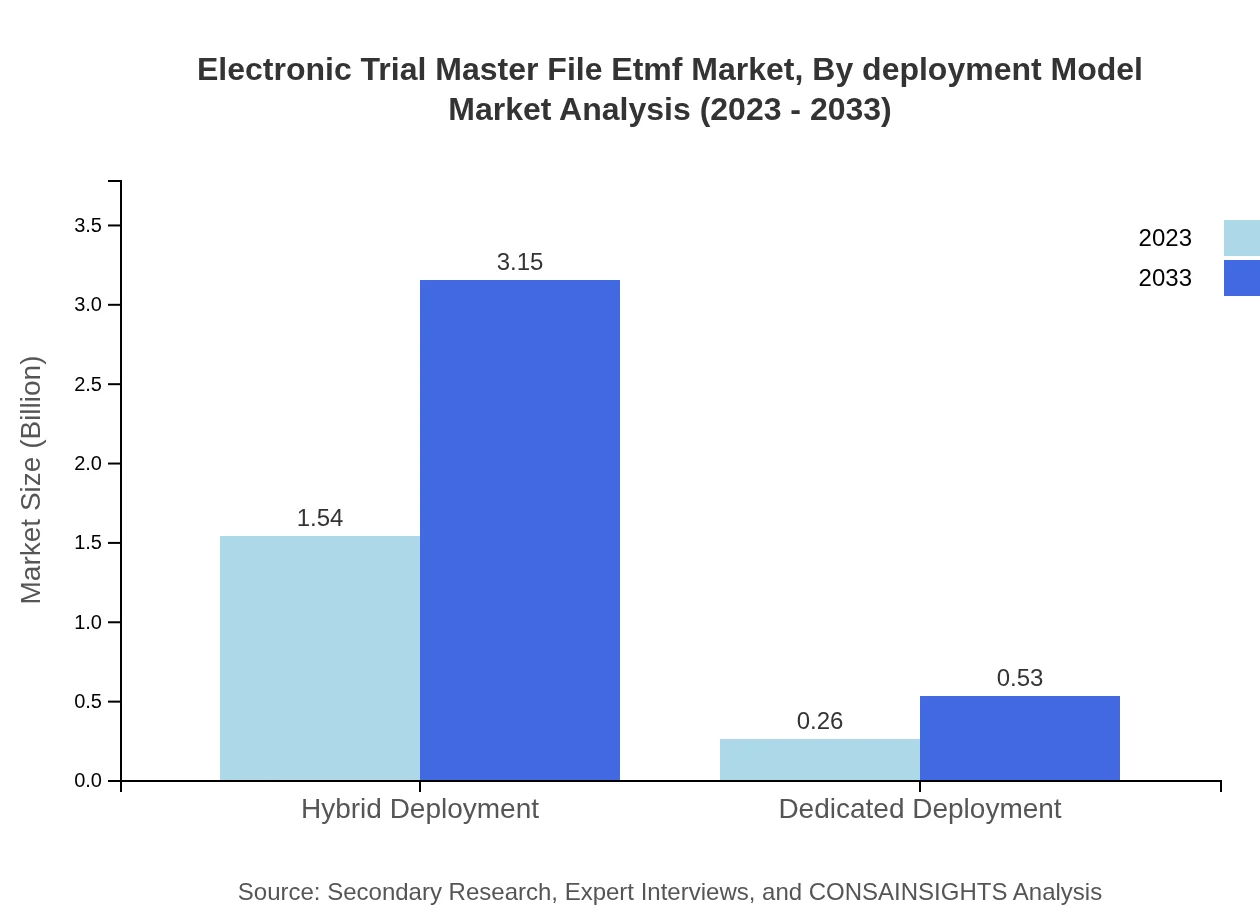
Electronic Trial Master File Etmf Market Analysis By Deployment Model
A notable trend is seen in the adoption of hybrid deployment models, which allow organizations flexibility and scalability. This segment is expected to grow significantly as companies look for customized solutions. Dedicated deployments, while smaller, are also showing increased investment.
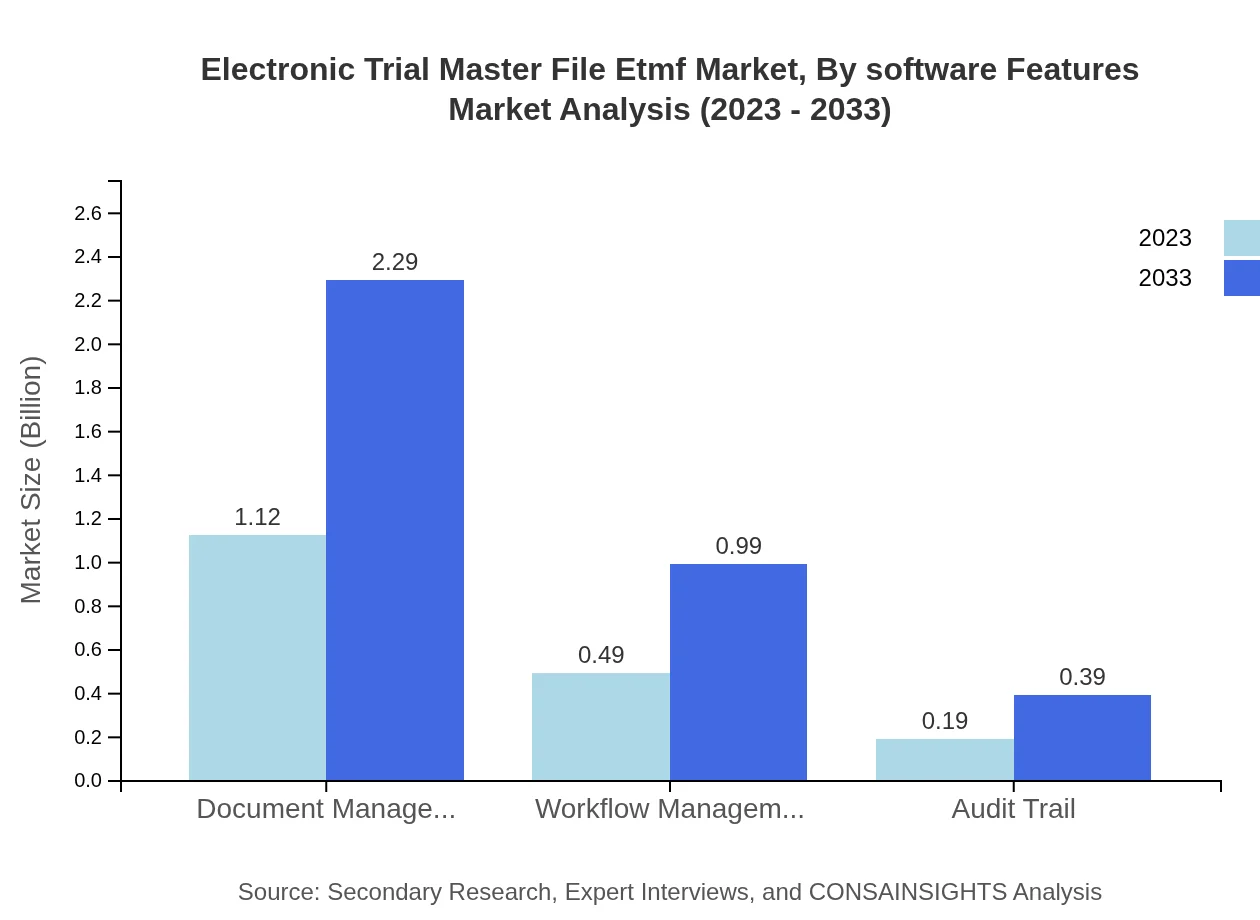
Electronic Trial Master File Etmf Market Analysis By Software Features
Document management and workflow management are crucial features driving market performance, with significant investments focused on enhancing functionalities such as audit trails and data access solutions to meet evolving industry demands.
Electronic Trial Master File Etmf Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Electronic Trial Master File Etmf Industry
Veeva Systems:
A leader in cloud-based solutions for life sciences, Veeva Systems offers the Veeva Vault eTMF, designed to streamline clinical trials and ensure regulatory compliance.Medidata Solutions:
Known for its cloud-based clinical development solutions, Medidata provides the Medidata eTMF system, facilitating efficient management of trial documentation.Oracle Corporation:
Through its comprehensive clinical data management suite, Oracle offers eTMF solutions that enhance collaboration and ensure compliance in clinical trials.TransCelerate BioPharma:
A collaborative initiative of biopharmaceutical companies aimed at simplifying and improving the clinical trial process through solutions like the eTMF Framework.MasterControl:
MasterControl offers quality management software that integrates seamlessly with eTMF to provide a comprehensive solution for managing clinical processes.We're grateful to work with incredible clients.









FAQs
What is the market size of electronic trial master file (eTMF)?
The electronic trial master file (eTMF) market size is projected to reach $1.8 billion by 2033, growing at a CAGR of 7.2% from 2023. This growth reflects the increasing need for efficient document management in clinical trials.
What are the key market players or companies in the electronic trial master file (eTMF) industry?
Key players in the eTMF market include major pharmaceutical firms, biotechnology companies, and clinical research organizations. These companies are crucial for driving innovation and adoption of eTMF solutions in clinical trials.
What are the primary factors driving the growth in the electronic trial master file (eTMF) industry?
Key drivers include the rising demand for efficient data management in clinical trials, regulatory compliance necessities, and the push towards digital solutions in healthcare, enhancing the efficiency of trial processes.
Which region is the fastest Growing in the electronic trial master file (eTMF)?
North America is currently the fastest-growing region for the eTMF market, projected to increase from $0.62 billion in 2023 to $1.26 billion by 2033, reflecting a strong growth potential driven by healthcare advancements.
Does ConsaInsights provide customized market report data for the electronic trial master file (eTMF) industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the eTMF industry, providing insights that help stakeholders make informed decisions based on personalized market analysis.
What deliverables can I expect from this electronic trial master file (eTMF) market research project?
Deliverables typically include a comprehensive market report, detailed regional and segment analysis, market forecasts, competitive landscapes, and strategic insights that provide valuable guidance for stakeholders.
What are the market trends of the electronic trial master file (eTMF)?
Recent trends indicate a shift towards cloud-based solutions, with a projected market share of 85.58% by 2033, emphasizing the need for scalable, flexible eTMF options that enhance collaboration and data accessibility.