Embolic Protection Devices Market Report
Published Date: 31 January 2026 | Report Code: embolic-protection-devices
Embolic Protection Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive market analysis of Embolic Protection Devices, including current trends, market size forecasts from 2023 to 2033, and detailed insights across various segments and regions of the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
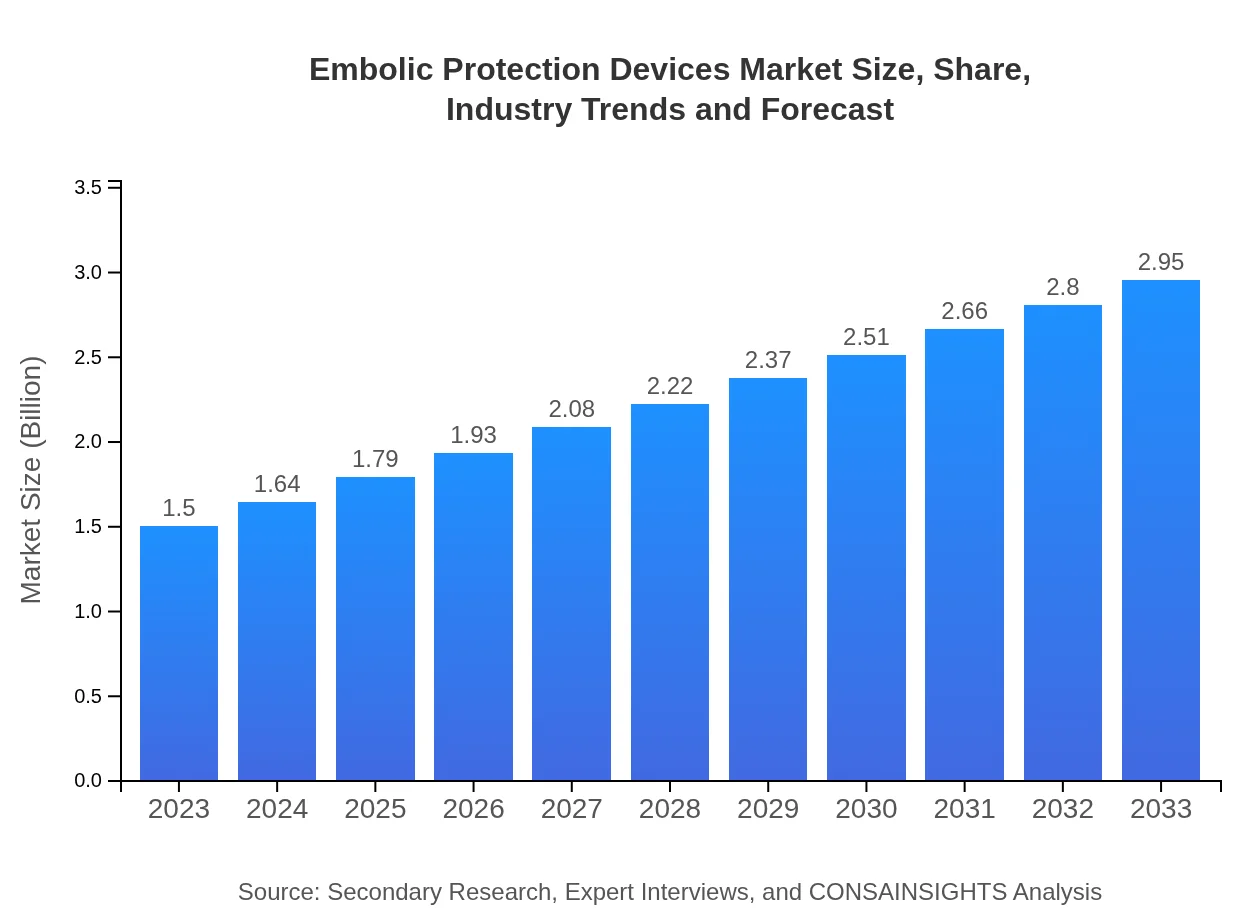
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Medtronic , Boston Scientific, Terumo, Abbott Laboratories |
| Last Modified Date | 31 January 2026 |
Embolic Protection Devices Market Overview
Customize Embolic Protection Devices Market Report market research report
- ✔ Get in-depth analysis of Embolic Protection Devices market size, growth, and forecasts.
- ✔ Understand Embolic Protection Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Embolic Protection Devices
What is the Market Size & CAGR of Embolic Protection Devices market in 2023?
Embolic Protection Devices Industry Analysis
Embolic Protection Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Embolic Protection Devices Market Analysis Report by Region
Europe Embolic Protection Devices Market Report:
The European market for Embolic Protection Devices is expected to grow from $0.54 billion in 2023 to $1.06 billion in 2033. A robust regulatory framework, along with an increasing prevalence of chronic diseases, supports market growth in the region.Asia Pacific Embolic Protection Devices Market Report:
The Asia Pacific region is expected to see significant growth in the Embolic Protection Devices market, estimated to rise from $0.27 billion in 2023 to $0.52 billion by 2033. Increasing urbanization and improved healthcare infrastructure are contributing to this rise, alongside growing awareness of vascular health.North America Embolic Protection Devices Market Report:
North America remains a leading market in the Embolic Protection Devices segment, with a market size projected to grow from $0.50 billion in 2023 to $0.98 billion in 2033. Increasing incidences of cardiovascular diseases and the high adoption rate of innovative medical devices drive this growth, coupled with strong investment in healthcare infrastructure.South America Embolic Protection Devices Market Report:
In South America, the market for Embolic Protection Devices, although smaller, is anticipated to grow from $0.02 billion in 2023 to $0.04 billion by 2033. The region's expanding healthcare market and the adoption of advanced medical technologies are key growth drivers.Middle East & Africa Embolic Protection Devices Market Report:
In the Middle East and Africa, the market is projected to reach $0.18 billion in 2023 and grow to $0.35 billion by 2033. Factors such as rising healthcare expenditure and the increasing prevalence of lifestyle diseases are boosting demand for Embolic Protection Devices.Tell us your focus area and get a customized research report.
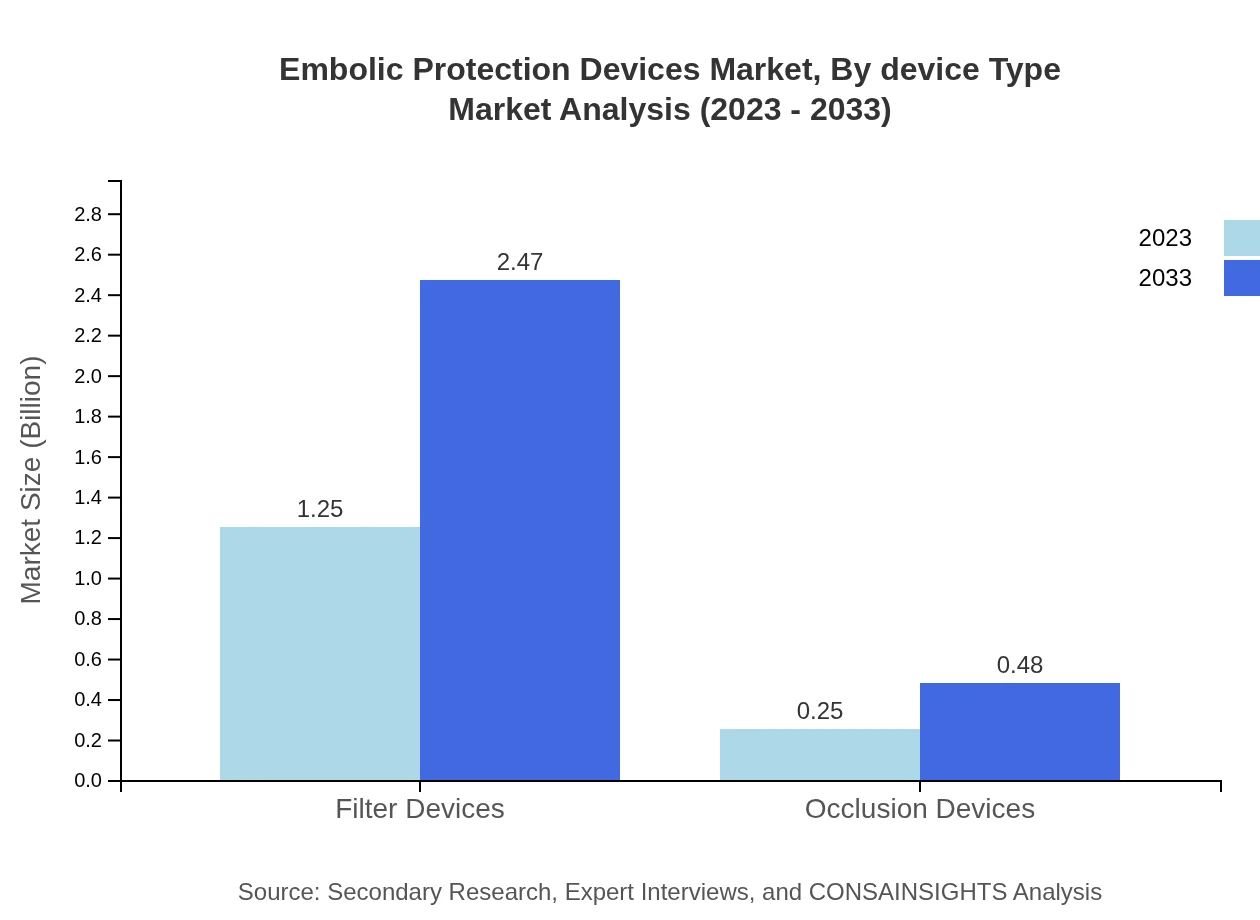
Embolic Protection Devices Market Analysis By Device Type
The market segmentation reveals that filter devices dominate the Embolic Protection Devices market, with a market size boosting from $1.25 billion in 2023 to $2.47 billion by 2033, capturing approximately 83.66% of the market share. Other segments include occlusion devices, which are also gaining traction, showcasing growth in line with the rising adoption of less invasive surgical approaches.
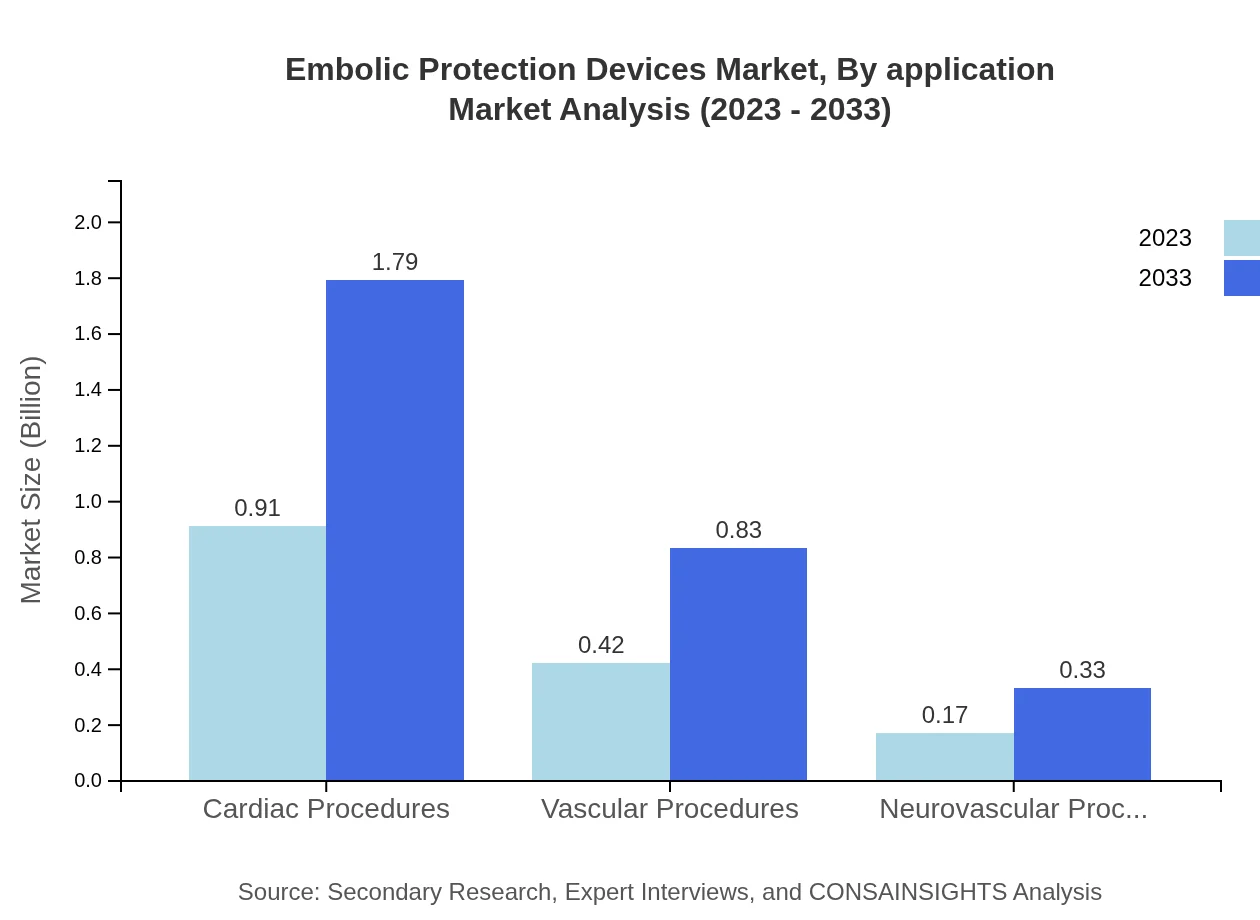
Embolic Protection Devices Market Analysis By Application
The application-based segmentation indicates that cardiac procedures lead the market, expected to grow from $0.91 billion in 2023 to $1.79 billion by 2033, maintaining a market share of around 60.79%. Vascular and neurovascular applications are also significant, with expected growth reflecting increased surgical activity in these areas.
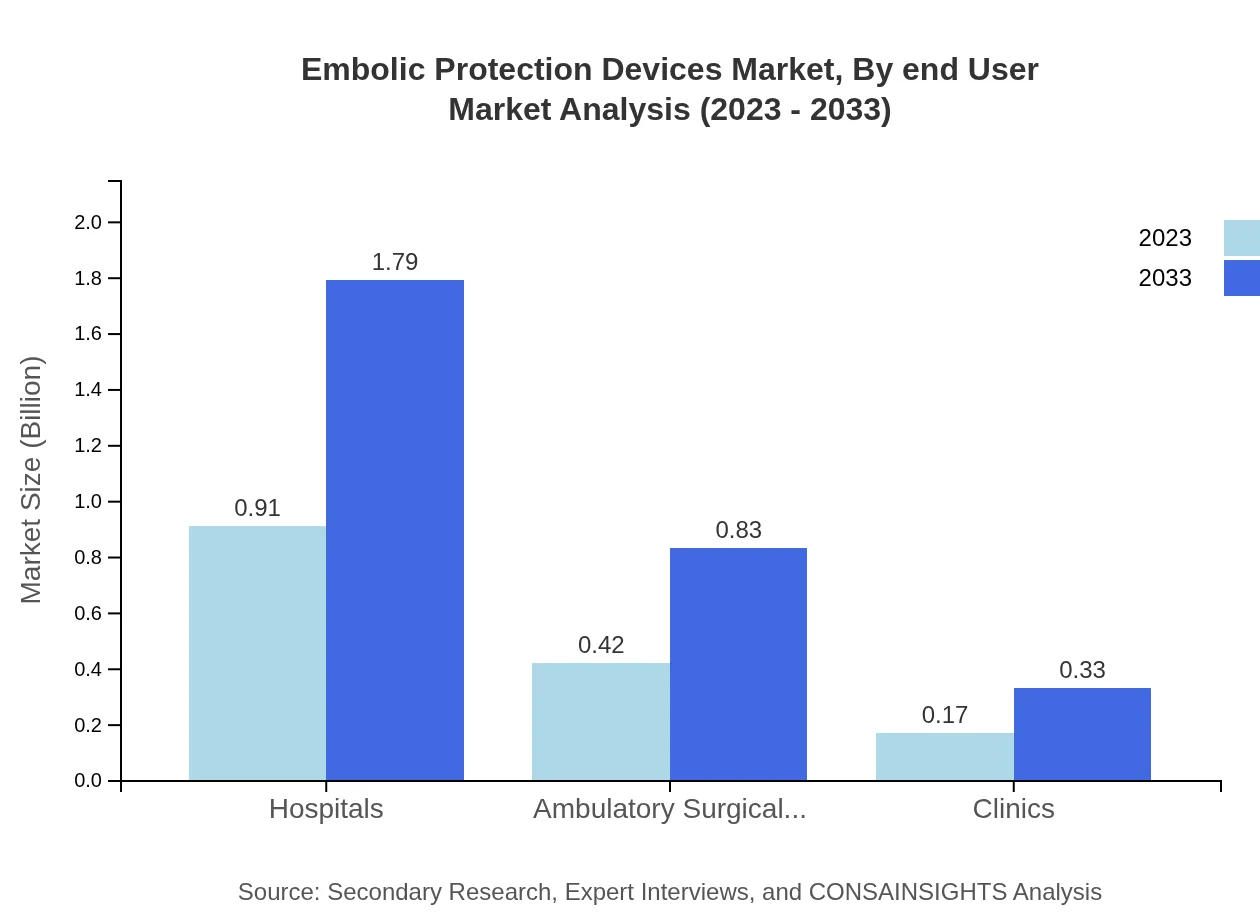
Embolic Protection Devices Market Analysis By End User
Hospitals are the primary end-users within the Embolic Protection Devices market, with a market size of $0.91 billion in 2023, expected to rise to $1.79 billion by 2033, retaining market dominance at 60.79%. Ambulatory surgical centers also show potential for growth, reflecting the increasing trend towards outpatient procedures.
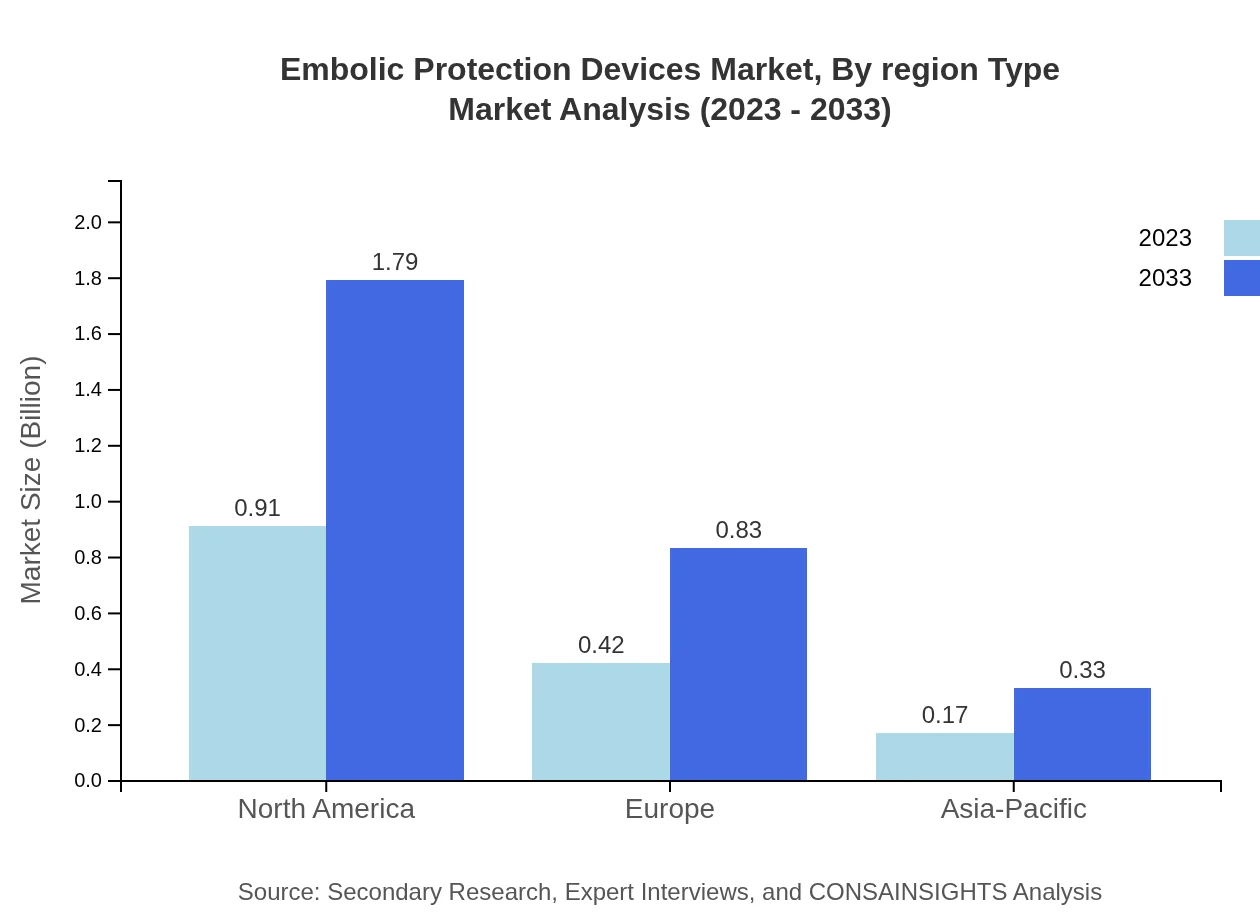
Embolic Protection Devices Market Analysis By Region Type
The comprehensive analysis of the regional segmentation showcases North America and Europe as the dominant markets due to developed healthcare infrastructures. In contrast, the Asia Pacific region, with its growing economy and improving healthcare access, is poised for the highest growth rate among emerging markets.
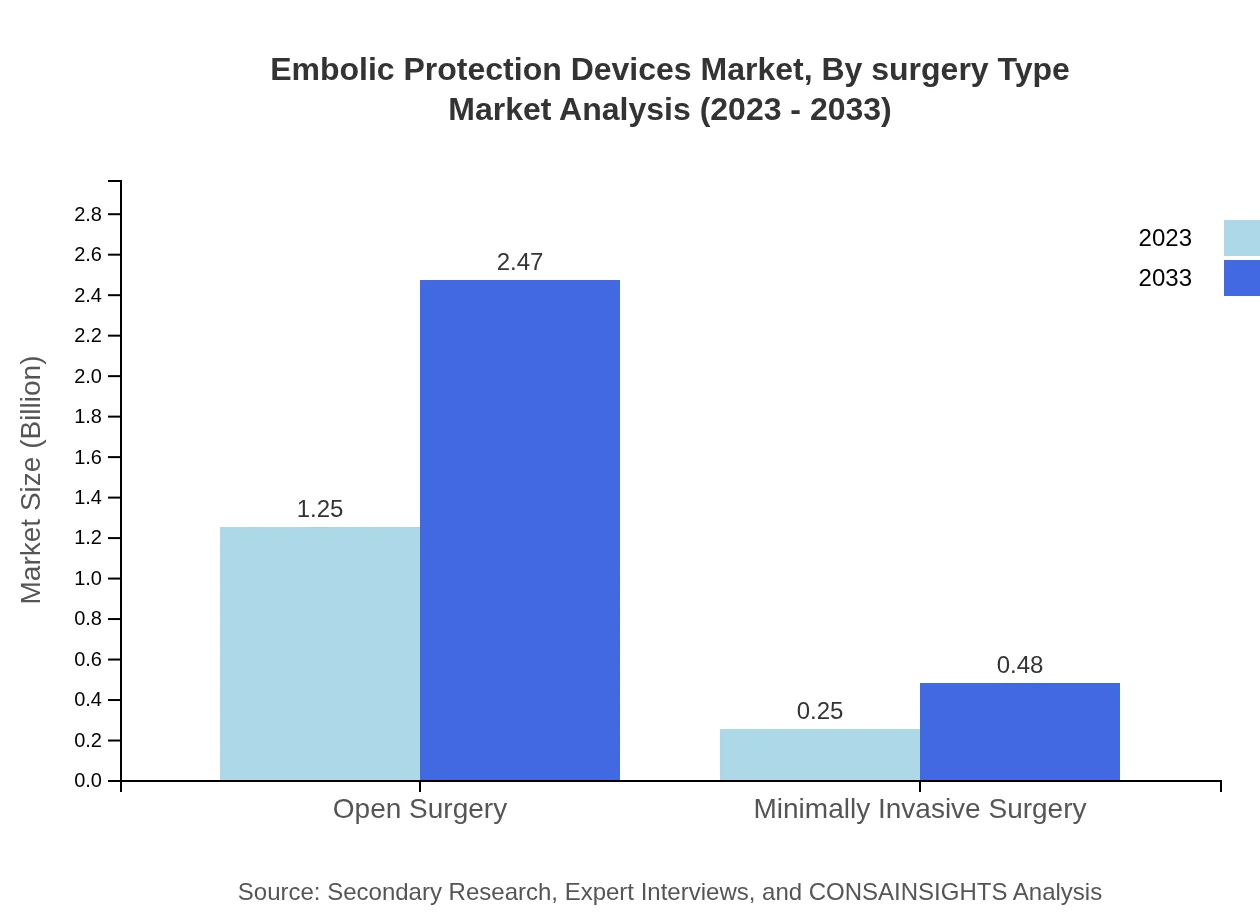
Embolic Protection Devices Market Analysis By Surgery Type
Open surgery is expected to maintain significant market capture, with size forecasted to increase from $1.25 billion in 2023 to $2.47 billion by 2033, reflecting reliability and established precedents in clinical practices. Minimally invasive surgery is also gaining favor, forecasted to grow correspondingly due to the associated benefits of faster recovery and reduced risk for patients.
Embolic Protection Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Embolic Protection Devices Industry
Medtronic :
A leader in the medical device industry, Medtronic offers a comprehensive range of Embolic Protection Devices and focuses heavily on research and innovation to improve patient outcomes.Boston Scientific:
Known for their advanced technological solutions, Boston Scientific provides various Embolic Protection Devices that cater to both surgical and procedural requirements across the globe.Terumo:
A key player providing innovative medical solutions, Terumo develops Embolic Protection Devices that enhance safety and efficiency during vascular procedures.Abbott Laboratories:
With a strong commitment to healthcare innovation, Abbott Laboratories offers a range of Embolic Protection Devices designed for high performance in demanding surgical environments.We're grateful to work with incredible clients.









FAQs
What is the market size of embolic protection devices?
The embolic protection devices market is projected to reach approximately $1.5 billion by 2033, growing at a CAGR of 6.8%. This growth reflects increasing demand for advanced medical technologies and improved patient outcomes in cardiovascular procedures.
What are the key market players or companies in this embolic protection devices industry?
Key players in the embolic protection devices market include Medtronic, Boston Scientific, and Abbott Laboratories. These companies are recognized for their innovative product offerings and strong market presence across various regions.
What are the primary factors driving the growth in the embolic protection devices industry?
Growth is driven by an increasing prevalence of cardiovascular diseases, rising geriatric populations, advancements in technology, and increased investments in healthcare infrastructure and research, enhancing the efficacy and safety of procedures.
Which region is the fastest Growing in the embolic protection devices market?
North America is currently the largest market for embolic protection devices, projected to grow from $0.91 billion in 2023 to $1.79 billion by 2033. Europe and Asia Pacific also show significant potential for growth as markets expand.
Does ConsaInsights provide customized market report data for the embolic protection devices industry?
Yes, ConsaInsights offers customized market report data for the embolic protection devices industry. Clients can request tailored insights based on specific criteria related to market trends, regional analysis, and competitive landscapes.
What deliverables can I expect from this embolic protection devices market research project?
From the embolic protection devices market research project, you can expect detailed reports encompassing market size forecasts, competitive analysis, segment analysis, growth drivers, trends, and regional insights, aiding strategic planning.
What are the market trends of embolic protection devices?
Current trends include the rising adoption of minimally invasive surgical techniques, an emphasis on patient safety and efficacy, technological advancements in device design, and an increasing focus on regulatory compliance and product innovation.