Engineered T Cells Market Report
Published Date: 31 January 2026 | Report Code: engineered-t-cells
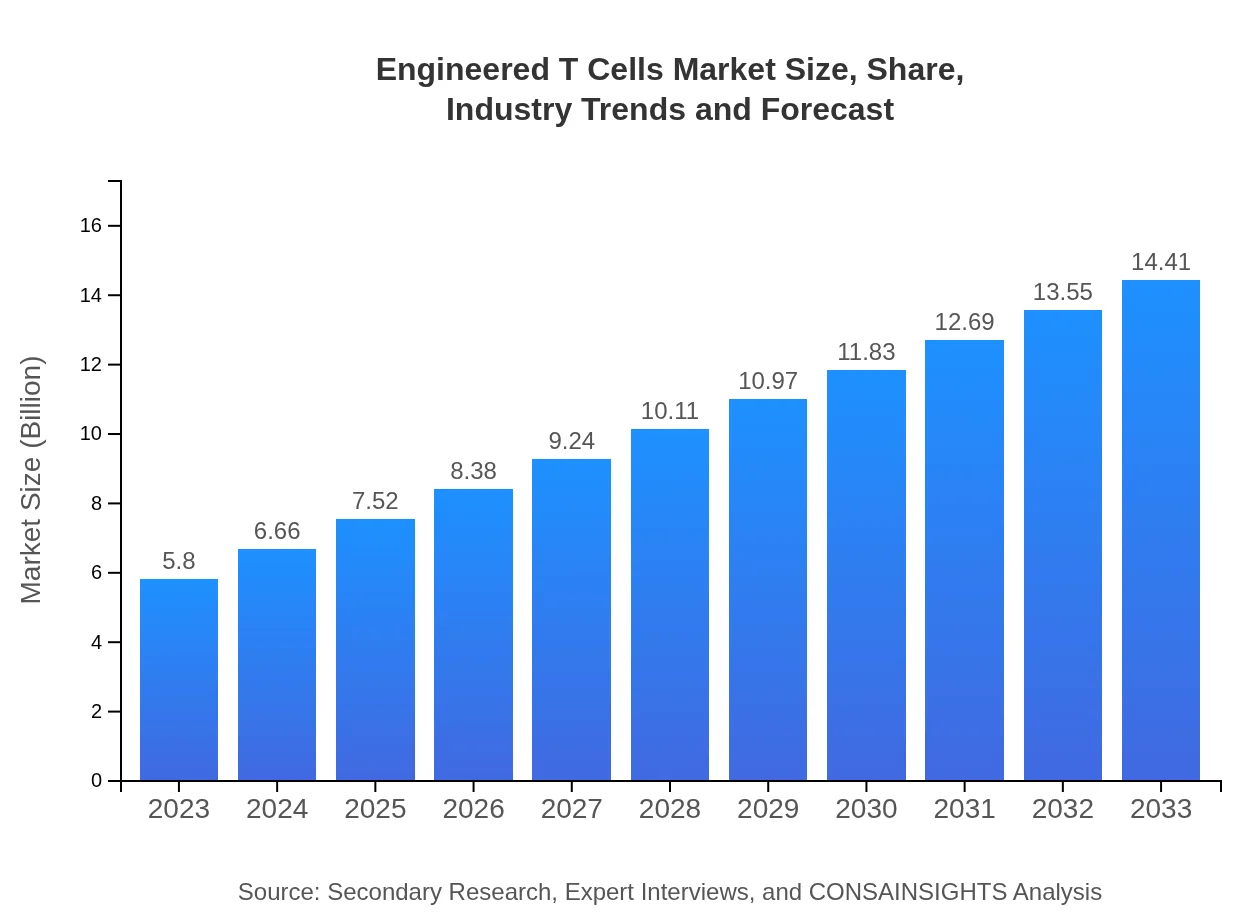
Engineered T Cells Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Engineered T Cells market, including current trends, market size, forecast data for 2023-2033, and insights into regional performances and technological advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.80 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $14.41 Billion |
| Top Companies | Novartis, Gilead Sciences, Bristol-Myers Squibb, Amgen, Regeneron Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Engineered T Cells Market Overview
Customize Engineered T Cells Market Report market research report
- ✔ Get in-depth analysis of Engineered T Cells market size, growth, and forecasts.
- ✔ Understand Engineered T Cells's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Engineered T Cells
What is the Market Size & CAGR of Engineered T Cells market in 2023?
Engineered T Cells Industry Analysis
Engineered T Cells Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Engineered T Cells Market Analysis Report by Region
Europe Engineered T Cells Market Report:
In Europe, the market size is projected to expand from $1.76 billion in 2023 to $4.37 billion by 2033. The European regulatory environment facilitates easier access to market for new therapies, while increasing prevalence rates of oncology cases drive demand.Asia Pacific Engineered T Cells Market Report:
The Asia Pacific region is expected to grow from $1.10 billion in 2023 to $2.72 billion by 2033, driven by robust healthcare investments and a booming biotechnology sector. Countries like China and Japan are leading the charge with substantial research output and clinical trial activities.North America Engineered T Cells Market Report:
North America dominates the Engineered T Cells market with an anticipated growth from $2.15 billion in 2023 to $5.34 billion by 2033. The region benefits from advanced healthcare infrastructure, high R&D investments, and the presence of leading biotech firms.South America Engineered T Cells Market Report:
In South America, the Engineered T Cells market is growing gradually, with projected values of $0.12 billion in 2023 to $0.29 billion by 2033. Growth factors include increasing healthcare accessibility and government initiatives to improve cancer treatment.Middle East & Africa Engineered T Cells Market Report:
The Middle East and Africa region estimates a market growth from $0.68 billion in 2023 to $1.69 billion by 2033, supported by a rising focus on advanced healthcare solutions and collaborations with global health organizations.Tell us your focus area and get a customized research report.
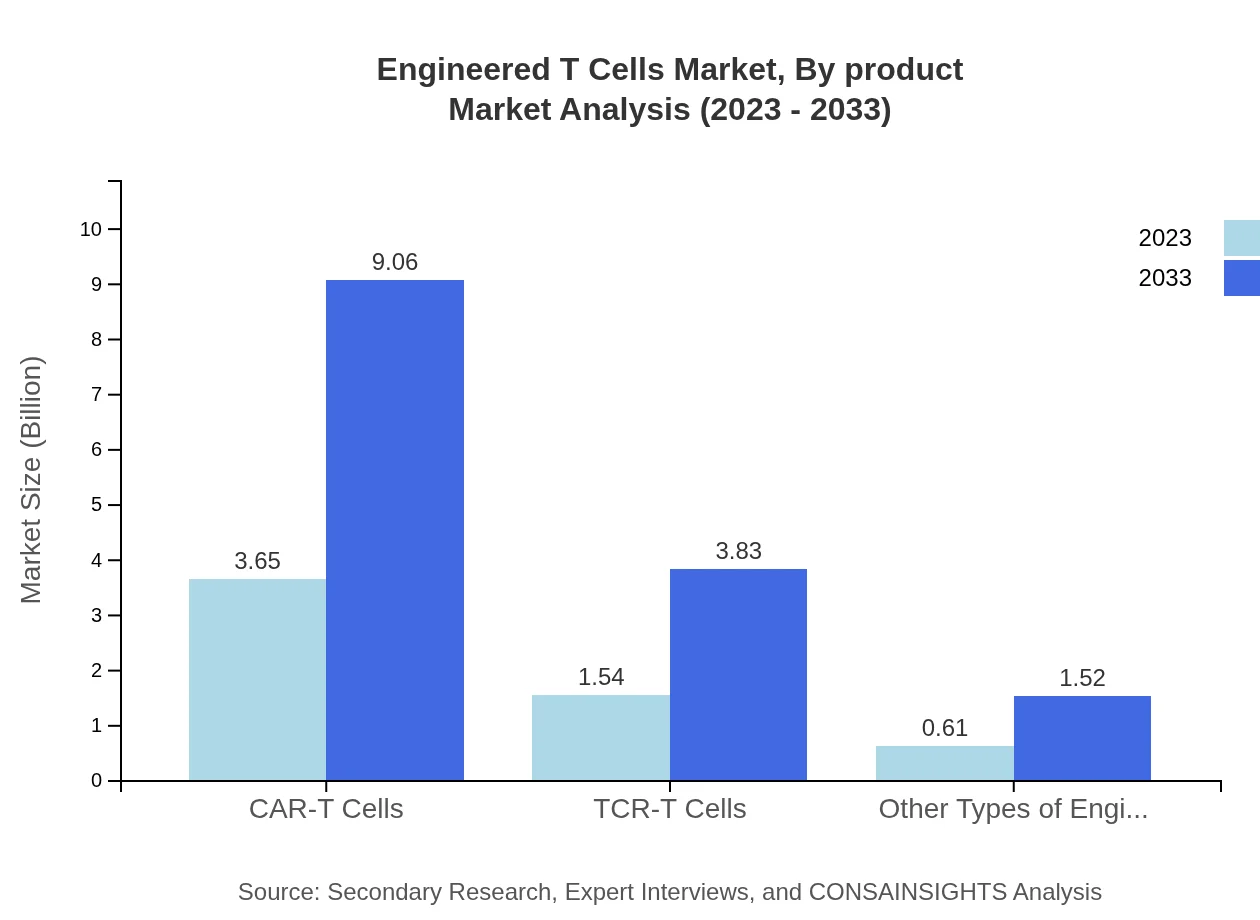
Engineered T Cells Market Analysis By Product
The Engineered T-Cells market is largely driven by CAR-T cells, expected to grow from $3.65 billion in 2023 to $9.06 billion by 2033. CAR-T therapy accounts for 62.87% of the market share, indicating its prominence. TCR-T cells follow, with a size of $1.54 billion in 2023, growing to $3.83 billion by 2033, making up 26.59% of the market. Other engineered T cells represent the remaining share, though they are gaining traction due to their applications in diverse therapeutic areas.
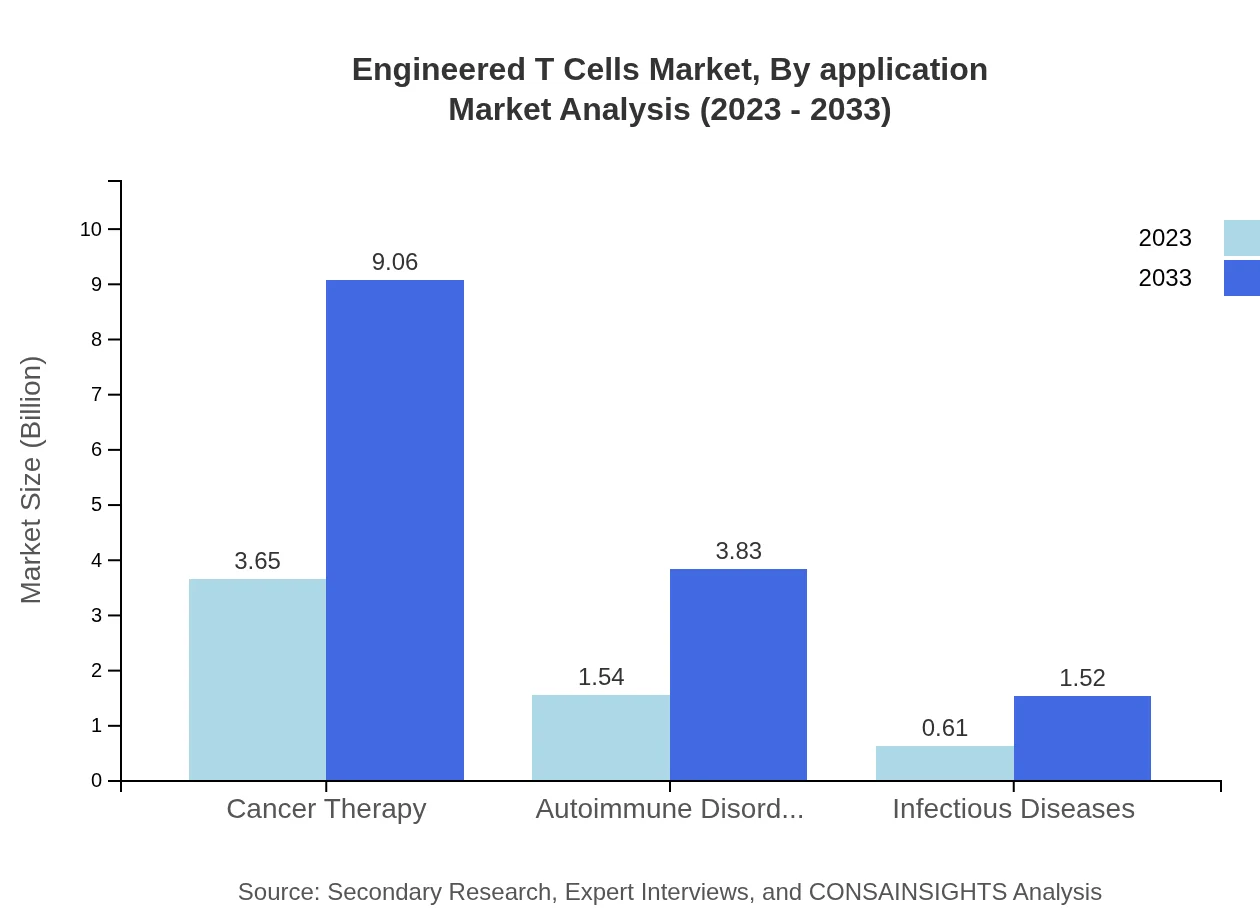
Engineered T Cells Market Analysis By Application
The predominant applications of Engineered T-Cells lie in Cancer Therapy, expected to rise from $3.65 billion in 2023 to $9.06 billion by 2033, representing a 62.87% market share. The treatment of Autoimmune Disorders and Infectious Diseases will grow significantly, from $1.54 billion to $3.83 billion (26.59% share) and from $0.61 billion to $1.52 billion (10.54% share), respectively.
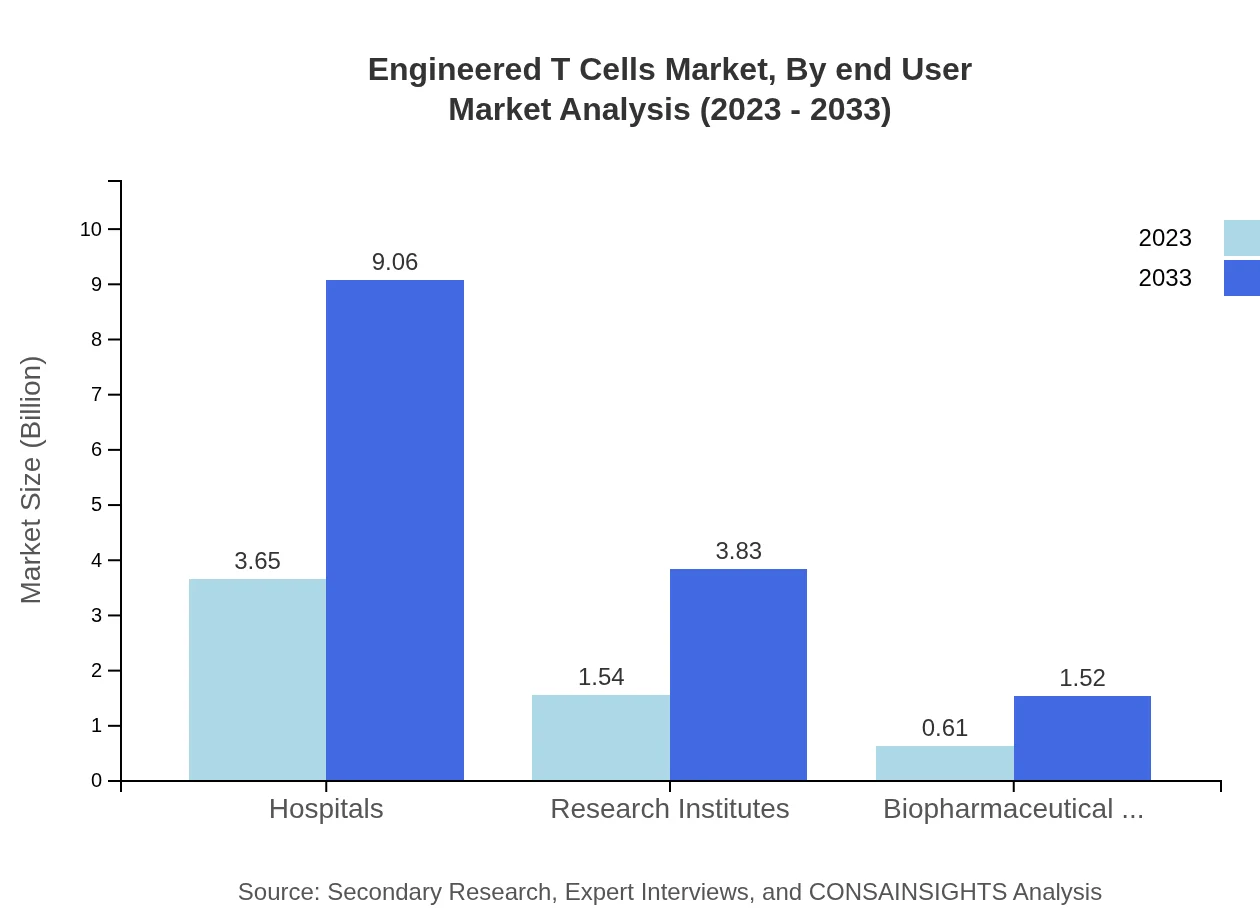
Engineered T Cells Market Analysis By End User
Hospitals are the primary end user of Engineered T-Cells, holding a market size of $3.65 billion in 2023, projected to reach $9.06 billion by 2033. Research institutes and biopharmaceutical companies play essential roles as well, with respective market sizes of $1.54 billion and $0.61 billion in 2023, indicating their contributions to advancing therapy research and development.
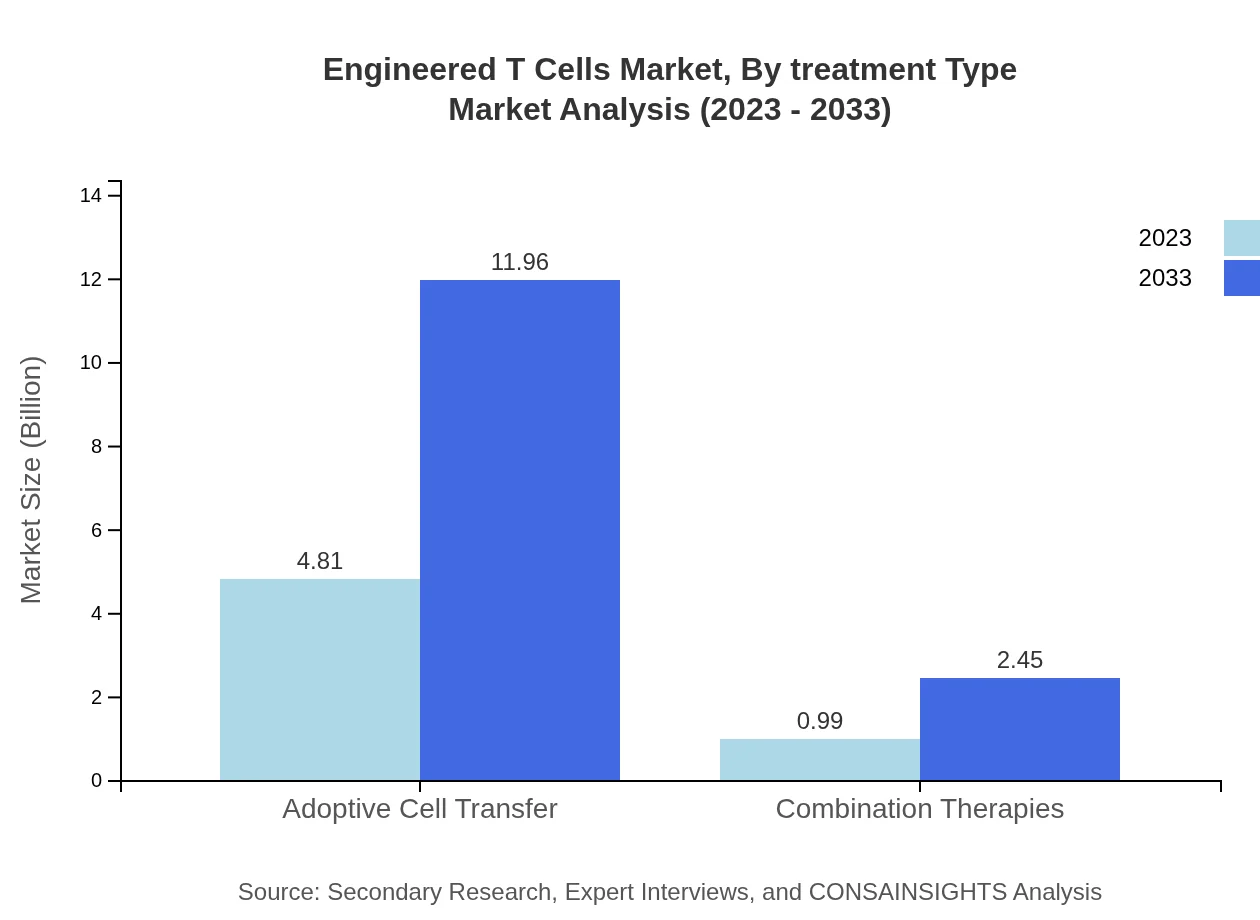
Engineered T Cells Market Analysis By Treatment Type
Adoptive Cell Transfer remains the leading treatment type, expanding from $4.81 billion in 2023 to $11.96 billion by 2033 (83% share). Combination Therapies also gain ground, projected to increase from $0.99 billion to $2.45 billion (17% share) as therapeutic strategies continue to evolve.
Engineered T Cells Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Engineered T Cells Industry
Novartis:
A pioneer in the development of CAR-T cell therapies, Novartis has transformed the treatment landscape for various cancer types, focusing on innovative approaches and global access.Gilead Sciences:
Renowned for its leadership in cell therapy, Gilead Sciences has extensive portfolios in engineered T cells and is dedicated to advancing biopharmaceutical technologies.Bristol-Myers Squibb:
Bristol-Myers Squibb is a major player in immuno-oncology, providing engineered T cell therapies that have received significant research and commercial success.Amgen:
Amgen is committed to the advancement of cell therapies, particularly in oncology and autoimmune disease treatment, backed by substantial research investments.Regeneron Pharmaceuticals:
Regeneron focuses on innovative immunotherapies, bringing engineered T cell therapies to market and promoting global health outreach initiatives.We're grateful to work with incredible clients.









FAQs
What is the market size of engineered T Cells?
The engineered T-cells market is currently valued at $5.8 billion in 2023 and is projected to grow at a CAGR of 9.2%, reaching significant milestones in the coming years.
What are the key market players or companies in the engineered T Cells industry?
Key players in the engineered T-cells industry include major biotechnology companies and research institutions focusing on cancer therapies, autoimmune diseases, and infectious diseases, prominently positioning themselves in the growing market.
What are the primary factors driving the growth in the engineered T Cells industry?
Primary growth drivers for engineered T-cells include advancements in cell therapy technologies, increased cancer prevalence, collaborations among biotech companies for research, and rising investments in innovative treatment solutions, enhancing patient outcomes.
Which region is the fastest Growing in the engineered T Cells?
The North American region is the fastest-growing market for engineered T-cells, projected to increase from $2.15 billion in 2023 to $5.34 billion by 2033, driven by robust healthcare infrastructure and research investments.
Does ConsaInsights provide customized market report data for the engineered T Cells industry?
Yes, ConsaInsights offers customized market report data tailored to the engineered T-cells industry, catering to specific client requirements and providing in-depth insights to facilitate informed business decisions.
What deliverables can I expect from this engineered T Cells market research project?
Expect comprehensive market analysis, including detailed reports, trends, forecasts, competitive landscape assessments, and tailored recommendations to enhance strategic planning in the engineered T-cells market.
What are the market trends of engineered T Cells?
Current market trends for engineered T-cells include increasing adoption in cancer therapies, growth in research initiatives focused on autoimmune disorders, and rising demand for combination therapies, highlighting sector expansion.