Enzyme Replacement Therapy Market Report
Published Date: 31 January 2026 | Report Code: enzyme-replacement-therapy
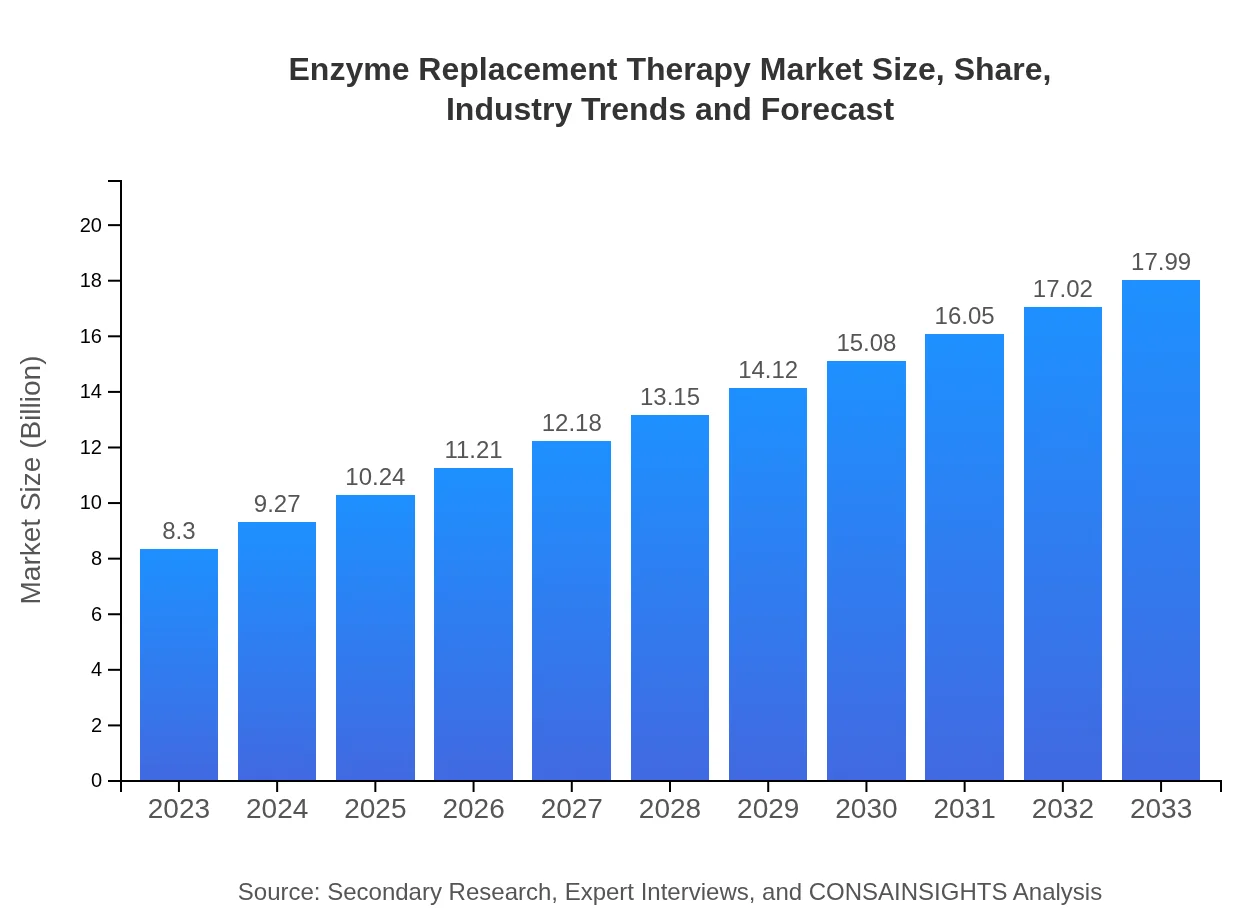
Enzyme Replacement Therapy Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive report analyzes the Enzyme Replacement Therapy market, providing insights on market size, trends, and forecasts from 2023 to 2033. The report covers regional analysis, industry segmentation, technology advancements, and key players, offering a holistic view of the evolving landscape of this crucial therapy.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $8.30 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $17.99 Billion |
| Top Companies | Genzyme Corporation (Sanofi), Shire Pharmaceuticals, Pfizer Inc., Bristol-Myers Squibb, Amicus Therapeutics |
| Last Modified Date | 31 January 2026 |
Enzyme Replacement Therapy Market Overview
Customize Enzyme Replacement Therapy Market Report market research report
- ✔ Get in-depth analysis of Enzyme Replacement Therapy market size, growth, and forecasts.
- ✔ Understand Enzyme Replacement Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Enzyme Replacement Therapy
What is the Market Size & CAGR of Enzyme Replacement Therapy market in 2023?
Enzyme Replacement Therapy Industry Analysis
Enzyme Replacement Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Enzyme Replacement Therapy Market Analysis Report by Region
Europe Enzyme Replacement Therapy Market Report:
The European market is expected to expand from USD 2.50 billion in 2023 to USD 5.42 billion by 2033. The growth is supported by increasing funding for biotechnology research and the presence of key market players in the region.Asia Pacific Enzyme Replacement Therapy Market Report:
The Asia Pacific region is expected to witness substantial growth, with the market size projected to increase from USD 1.61 billion in 2023 to USD 3.49 billion by 2033. This growth is driven by rising healthcare investments, an increase in metabolic disorder awareness, and improving healthcare infrastructure in countries like China and India.North America Enzyme Replacement Therapy Market Report:
North America remains the largest market for Enzyme Replacement Therapy, with the market size projected to grow from USD 2.93 billion in 2023 to USD 6.36 billion by 2033. The region benefits from advanced healthcare facilities, high awareness levels, and a robust pipeline of innovative therapies.South America Enzyme Replacement Therapy Market Report:
In South America, the Enzyme Replacement Therapy market is anticipated to expand from USD 0.82 billion in 2023 to USD 1.78 billion by 2033. Factors such as increasing prevalence of genetic disorders and growing investment in healthcare services are driving this growth.Middle East & Africa Enzyme Replacement Therapy Market Report:
The Middle East and Africa (MEA) region is projected to reach USD 0.95 billion by 2033, expanding from USD 0.44 billion in 2023. The growth in this region is hindered by economic challenges but is supported by increasing health awareness initiatives.Tell us your focus area and get a customized research report.
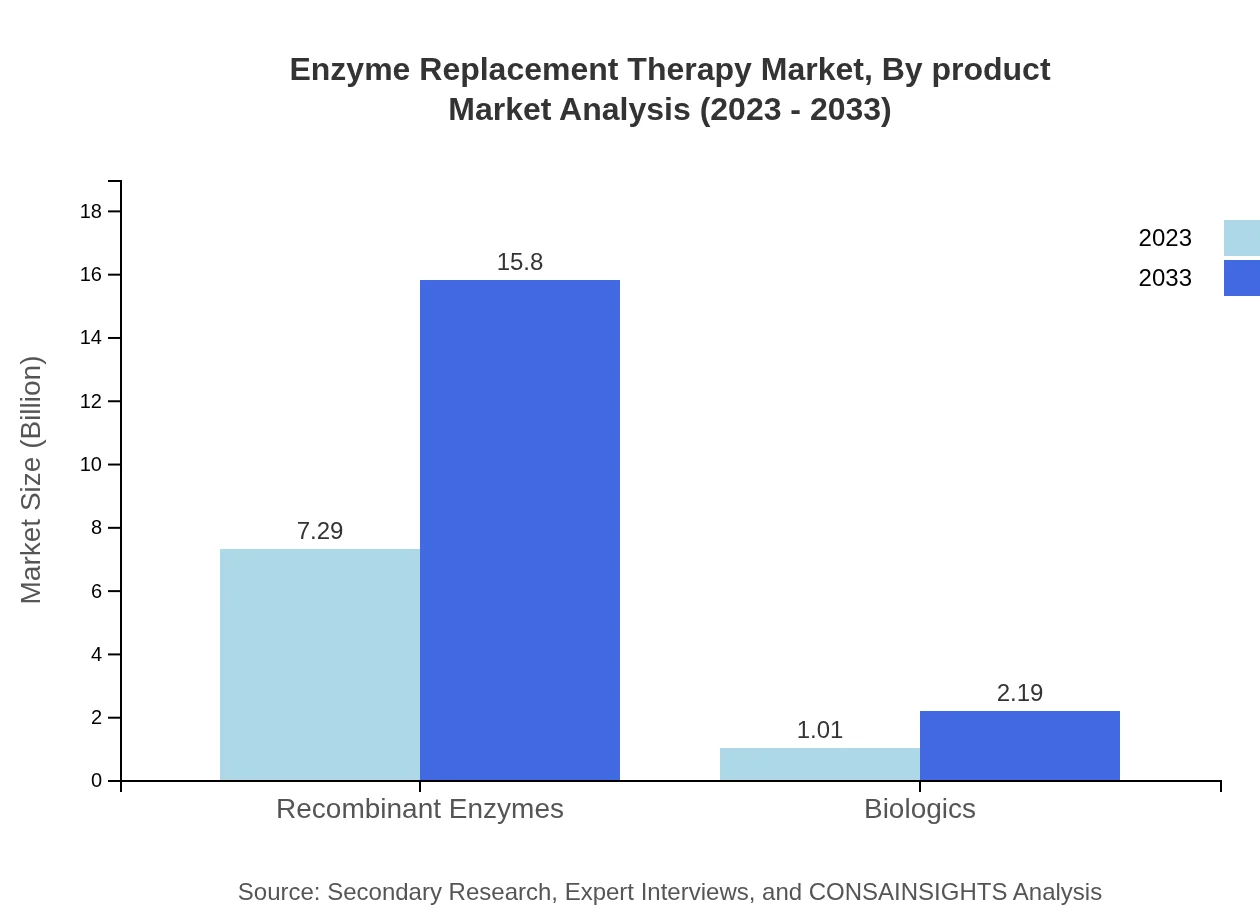
Enzyme Replacement Therapy Market Analysis By Product
The recombinant enzymes segment holds a significant share of the Enzyme Replacement Therapy market, with projected growth from USD 7.29 billion in 2023 to USD 15.80 billion by 2033. Biologics, though smaller in size, are expected to grow from USD 1.01 billion in 2023 to USD 2.19 billion by 2033, indicating its increasing significance.
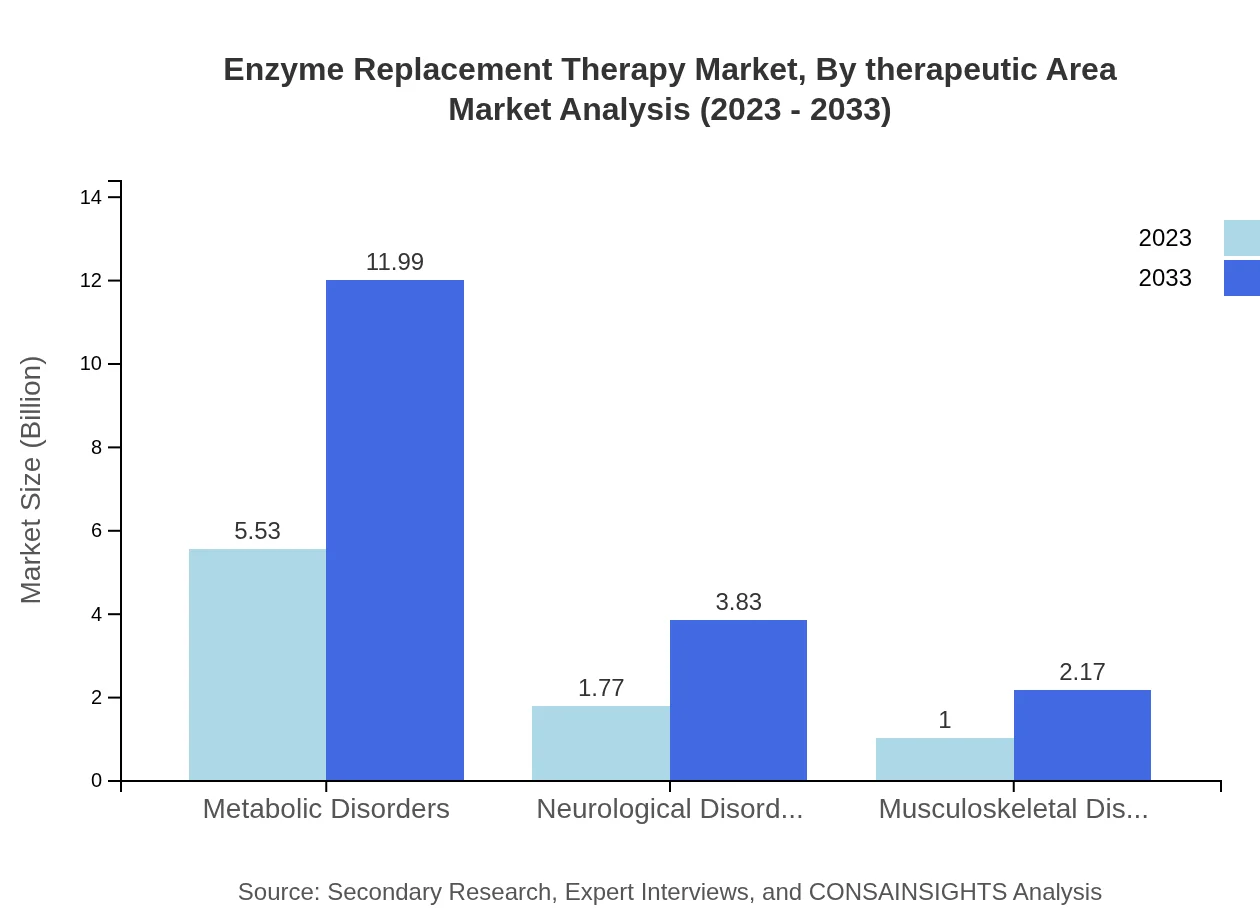
Enzyme Replacement Therapy Market Analysis By Therapeutic Area
The metabolic disorders segment dominates the therapeutic areas for ERT, expected to reach USD 11.99 billion by 2033 from USD 5.53 billion in 2023, highlighting its importance in treating genetic conditions. Neurological and musculoskeletal disorders also contribute significant market shares.
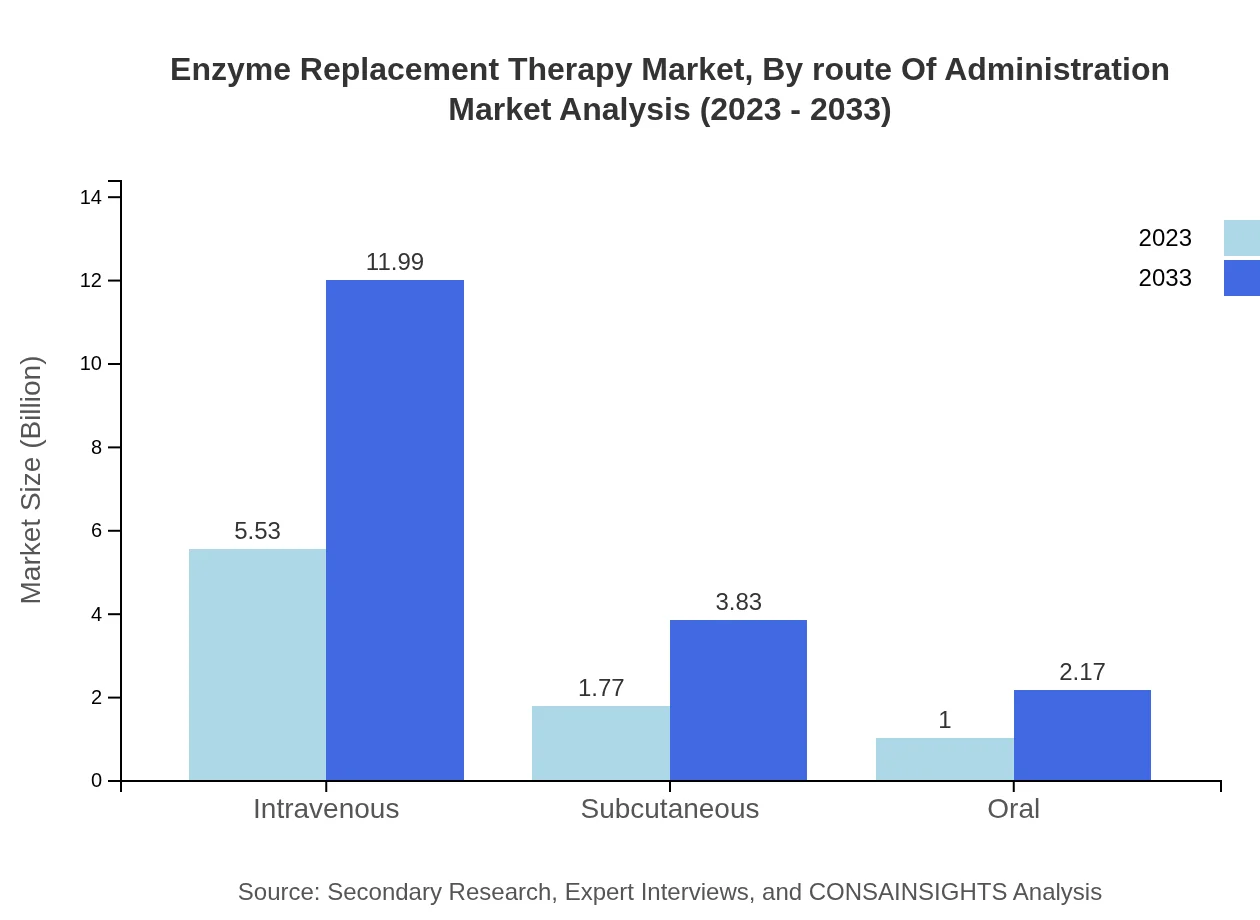
Enzyme Replacement Therapy Market Analysis By Route Of Administration
The intravenous route of administration demonstrates the largest share at 66.65%, with market size projected to increase from USD 5.53 billion in 2023 to USD 11.99 billion by 2033. Subcutaneous and oral administration routes are also growing, reaching USD 3.83 billion and USD 2.17 billion respectively by 2033.
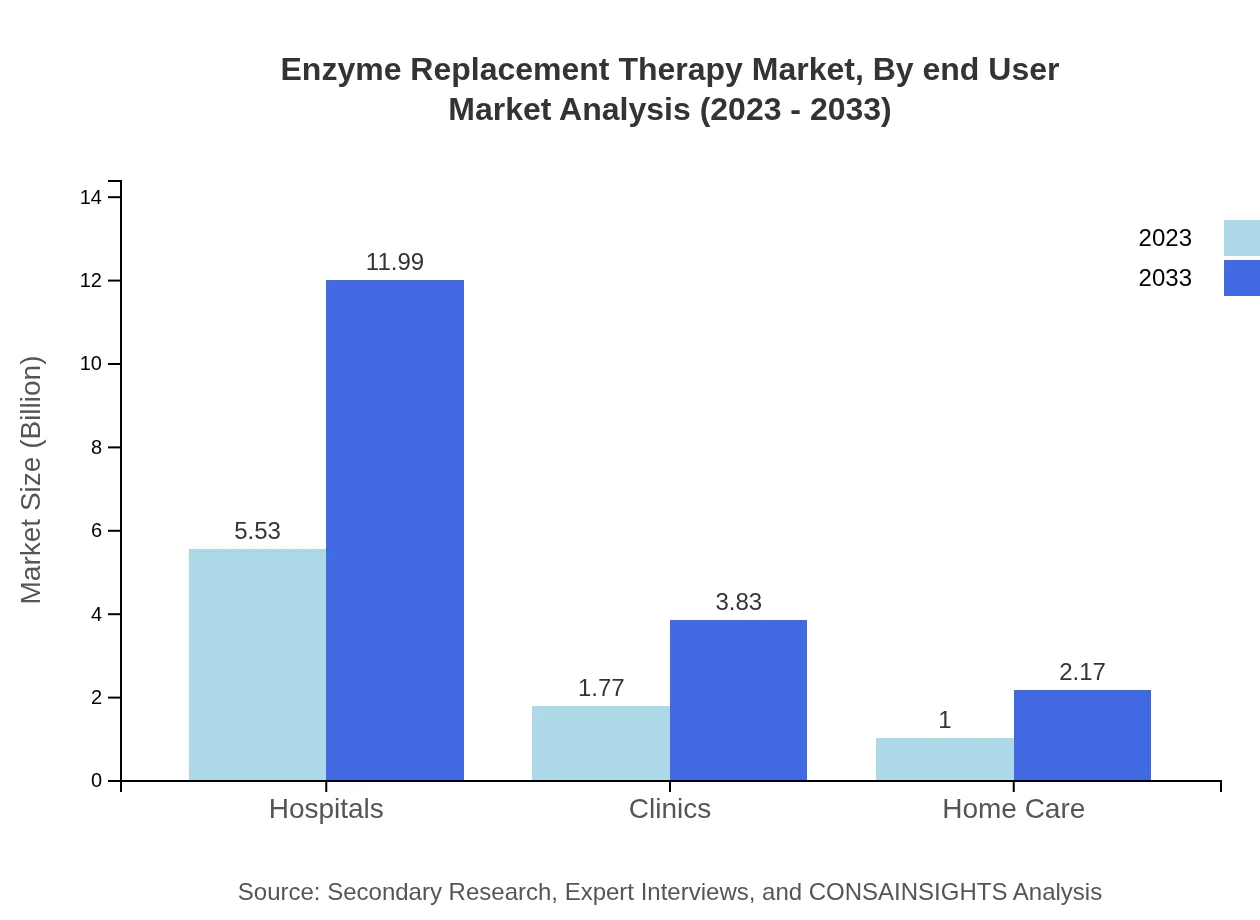
Enzyme Replacement Therapy Market Analysis By End User
Hospital pharmacies are the primary end-users, accounting for 66.65% of the market, with size growing from USD 5.53 billion in 2023 to USD 11.99 billion by 2033. Retail pharmacies and clinics also contribute significantly, serving a vital role in patient care.
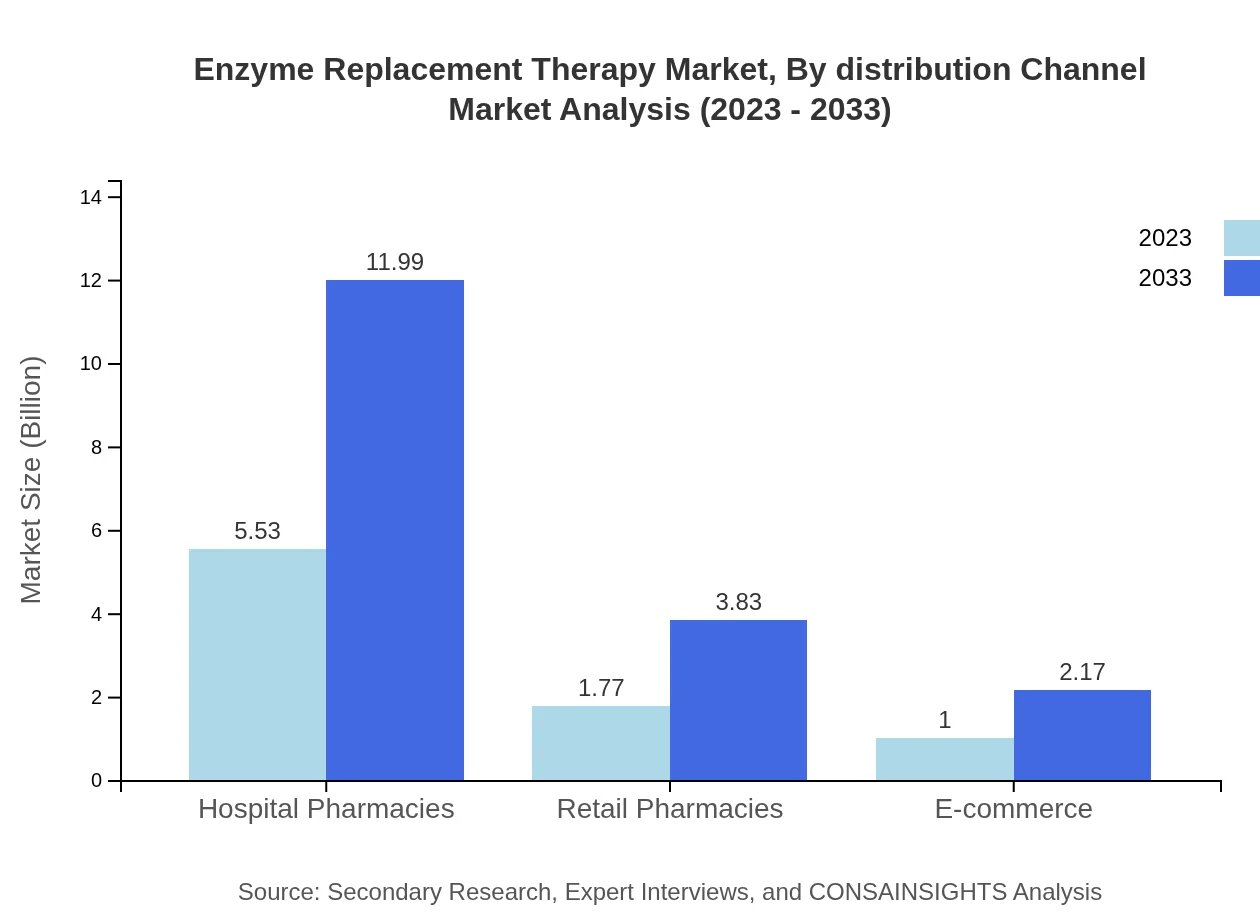
Enzyme Replacement Therapy Market Analysis By Distribution Channel
Distribution involves hospital pharmacies (66.65% share), retail pharmacies (21.31%), and e-commerce (12.04%), reflecting evolving purchasing preferences. These distribution channels affect market reach and accessibility for end-users.
Enzyme Replacement Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Enzyme Replacement Therapy Industry
Genzyme Corporation (Sanofi):
Focuses on providing innovative therapies for rare genetic diseases, leading the market with products such as Cerezyme and Fabrazyme.Shire Pharmaceuticals:
Known for its robust portfolio of ERT products, including Vpriv for Gaucher's disease, contributing significantly to industry growth.Pfizer Inc.:
Major player investing in ERT research, dedicated to expanding therapeutic options for patients with metabolic disorders.Bristol-Myers Squibb:
A leading biopharmaceutical company actively engaged in developing ERT solutions for rare diseases.Amicus Therapeutics:
Specializes in the development of ERTs and has recently achieved notable advancements in treatment options.We're grateful to work with incredible clients.









FAQs
What is the market size of enzyme Replacement Therapy?
The global enzyme replacement therapy market was valued at approximately $8.3 billion in 2023, with a projected CAGR of 7.8%, expected to reach a significantly higher value by 2033. This growth indicates increasing demand and advancements in treatment options.
What are the key market players or companies in this enzyme Replacement Therapy industry?
Key market players in the enzyme replacement therapy industry include major pharmaceutical companies such as Sanofi, Takeda Pharmaceutical, and Genzyme, all of which are focused on developing and distributing innovative therapies to treat a range of metabolic disorders.
What are the primary factors driving the growth in the enzyme Replacement Therapy industry?
Factors driving growth in the enzyme replacement therapy market include rising prevalence of genetic disorders, increased investment in R&D of biologics, and greater awareness and diagnosis of metabolic diseases that necessitate effective treatment options.
Which region is the fastest Growing in enzyme Replacement Therapy?
North America is the fastest-growing region in the enzyme replacement therapy market, projected to expand from $2.93 billion in 2023 to $6.36 billion in 2033, driven by advanced healthcare infrastructure and rising demand for novel therapies.
Does ConsaInsights provide customized market report data for the enzyme Replacement Therapy industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the enzyme replacement therapy industry, allowing clients to gain detailed insights relevant to their market segment and geographical areas of interest.
What deliverables can I expect from this enzyme Replacement Therapy market research project?
Clients can expect various deliverables from the enzyme replacement therapy market research project, including detailed market analysis, growth forecasts, competitive landscape assessments, and customized reports reflecting insights tailored to specific inquiry.
What are the market trends of enzyme Replacement Therapy?
Market trends in enzyme replacement therapy include a shift towards personalized medicine, increasing adoption of biologics, and advancing technologies in drug delivery, reflecting the sector's commitment to improved patient outcomes through tailored therapeutic approaches.