Epilepsy Drugs Market Report
Published Date: 31 January 2026 | Report Code: epilepsy-drugs
Epilepsy Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the epilepsy drugs market from 2023 to 2033, including market size, growth trends, industry analysis, segmentation, regional insights, and leading players along with future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
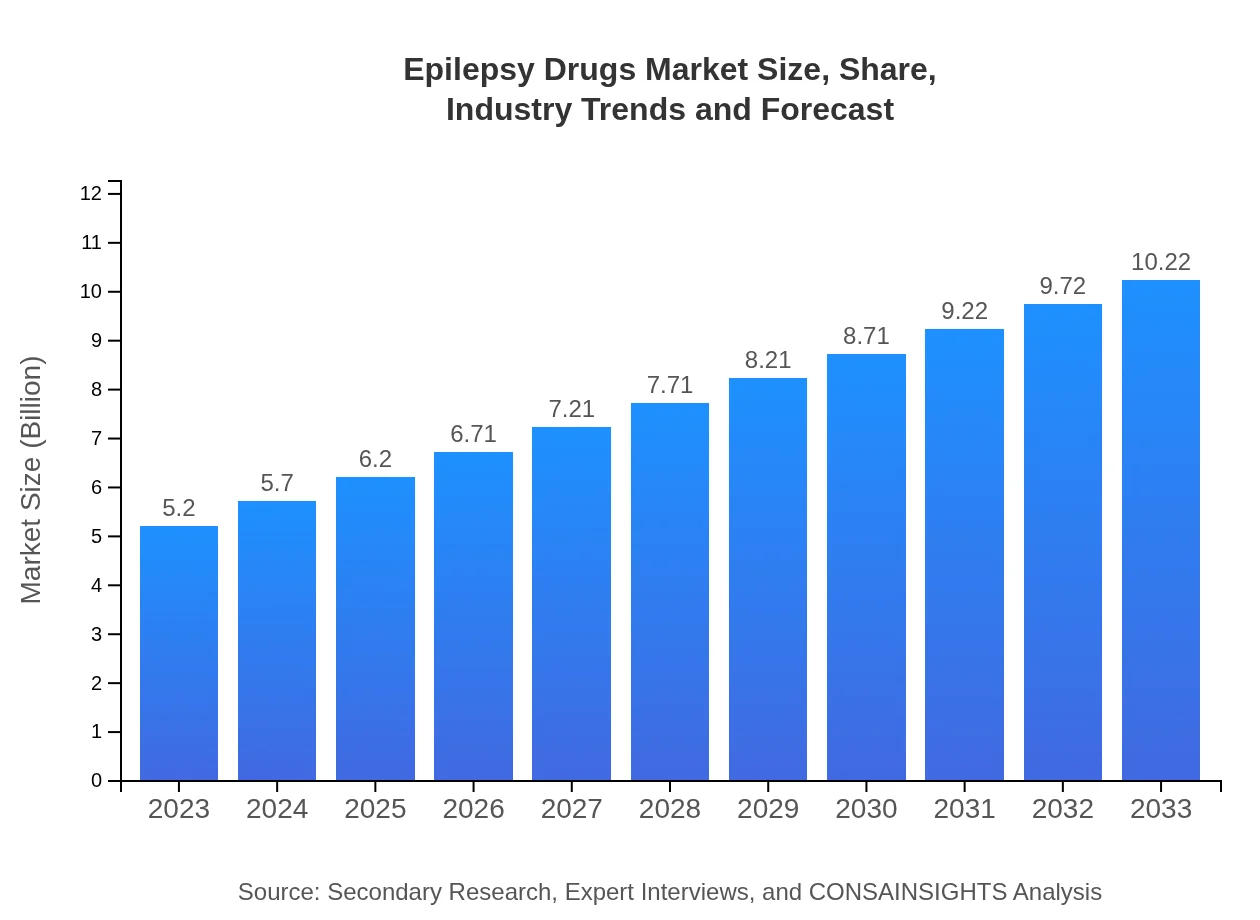
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Pfizer Inc., UCB S.A., Eisai Co., Ltd., Novartis AG, Sanofi S.A. |
| Last Modified Date | 31 January 2026 |
Epilepsy Drugs Market Overview
Customize Epilepsy Drugs Market Report market research report
- ✔ Get in-depth analysis of Epilepsy Drugs market size, growth, and forecasts.
- ✔ Understand Epilepsy Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Epilepsy Drugs
What is the Market Size & CAGR of Epilepsy Drugs market in 2023?
Epilepsy Drugs Industry Analysis
Epilepsy Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Epilepsy Drugs Market Analysis Report by Region
Europe Epilepsy Drugs Market Report:
Europe's epilepsy drugs market was valued at $1.73 billion in 2023, with projections reaching $3.40 billion by 2033. The region is characterized by strong regulatory frameworks and substantial investments in healthcare, which support market advancement.Asia Pacific Epilepsy Drugs Market Report:
In the Asia Pacific region, the epilepsy drugs market was valued at approximately $0.94 billion in 2023, projected to grow to $1.85 billion by 2033. Enhanced healthcare access, growing awareness of epilepsy, and government initiatives supporting epilepsy treatment are contributors to this growth.North America Epilepsy Drugs Market Report:
North America dominated the market with a value of $1.78 billion in 2023, forecasted to rise to $3.50 billion by 2033. Factors like a high prevalence of epilepsy, advanced healthcare systems, and substantial R&D investments by key players in the region drive this robust growth.South America Epilepsy Drugs Market Report:
The South American market is smaller, estimated at $0.50 billion in 2023 and expected to reach $0.99 billion by 2033. Increasing urbanization and improving healthcare infrastructure are critical factors fueling market growth in this region.Middle East & Africa Epilepsy Drugs Market Report:
The market in the Middle East and Africa is relatively smaller, valued at $0.24 billion in 2023, with expectations to grow to $0.47 billion by 2033. Economic development and increased healthcare investment are essential for growth in this region.Tell us your focus area and get a customized research report.
Epilepsy Drugs Market Analysis By Drug Type
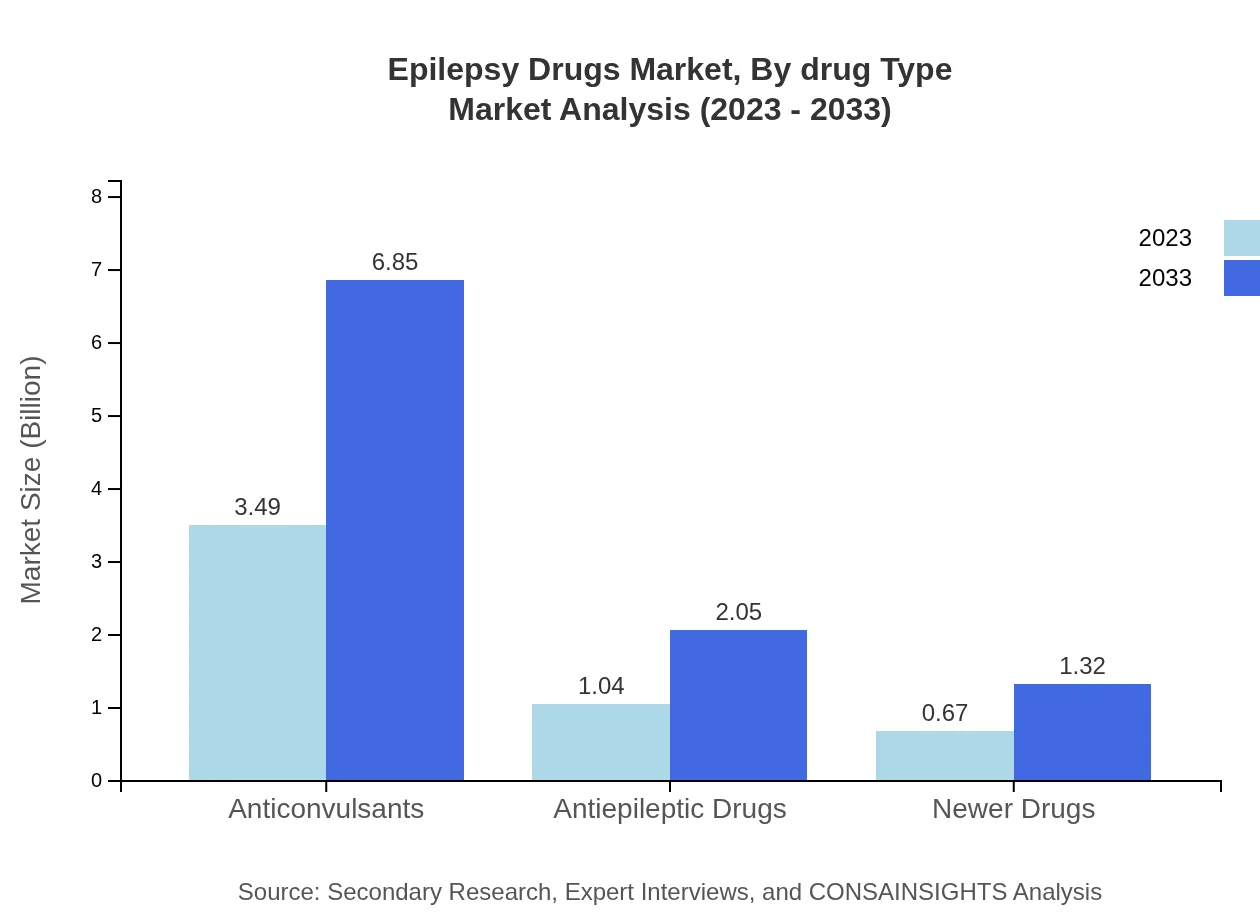
The Epilepsy Drugs Market by Drug Type reveals significant distinctions in performance. Anticonvulsants dominate this sector, expected to grow from $3.49 billion in 2023 to $6.85 billion by 2033, holding a market share of 67.07%. In contrast, antiepileptic drugs will grow from $1.04 billion to $2.05 billion (20.02% share), while newer drugs will expand from $0.67 billion to $1.32 billion, maintaining a 12.91% share.
Epilepsy Drugs Market Analysis By Therapy Type
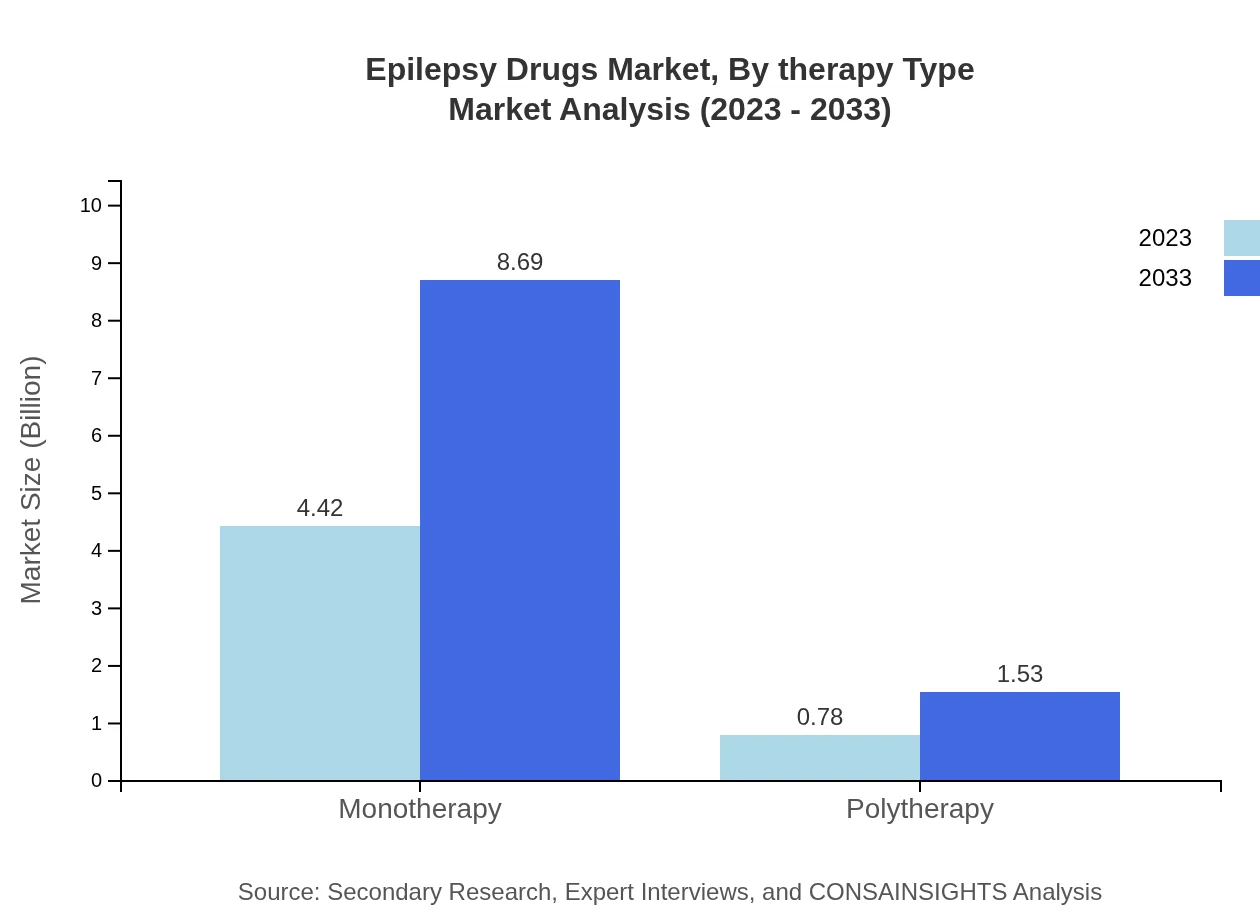
When analyzing by therapy type, the market is predominantly driven by monotherapy, which will grow from $4.42 billion in 2023 to $8.69 billion by 2033, constituting 85.03% share. Polytherapy, while less prevalent, is expected to increase from $0.78 billion to $1.53 billion, holding a 14.97% share.
Epilepsy Drugs Market Analysis By Route Of Administration
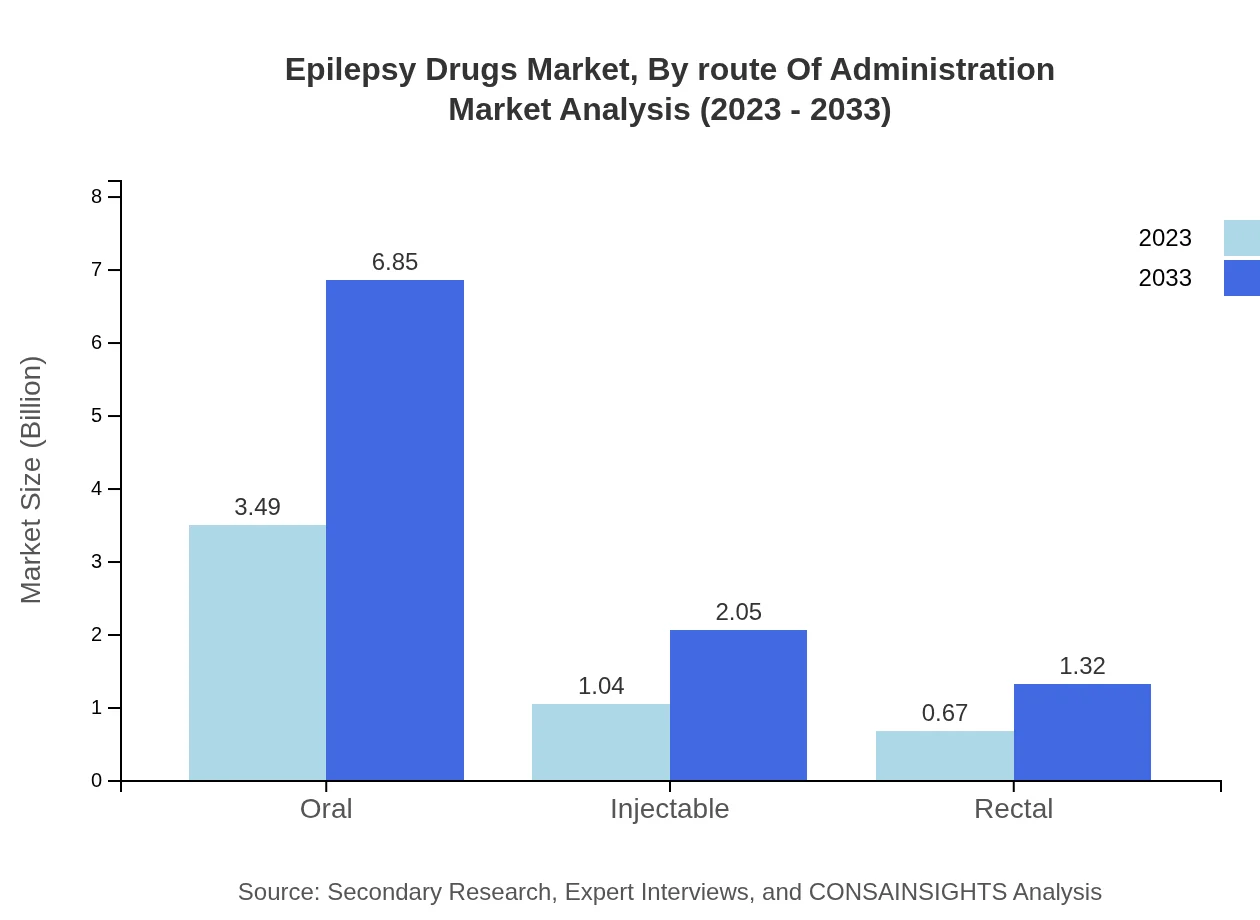
The oral route of administration leads the epilepsy drugs market, projected to grow from $3.49 billion in 2023 to $6.85 billion by 2033, maintaining a 67.07% market share. Injectable forms will rise from $1.04 billion to $2.05 billion (20.02% share), while rectal formulations will expand from $0.67 billion to $1.32 billion (12.91% share).
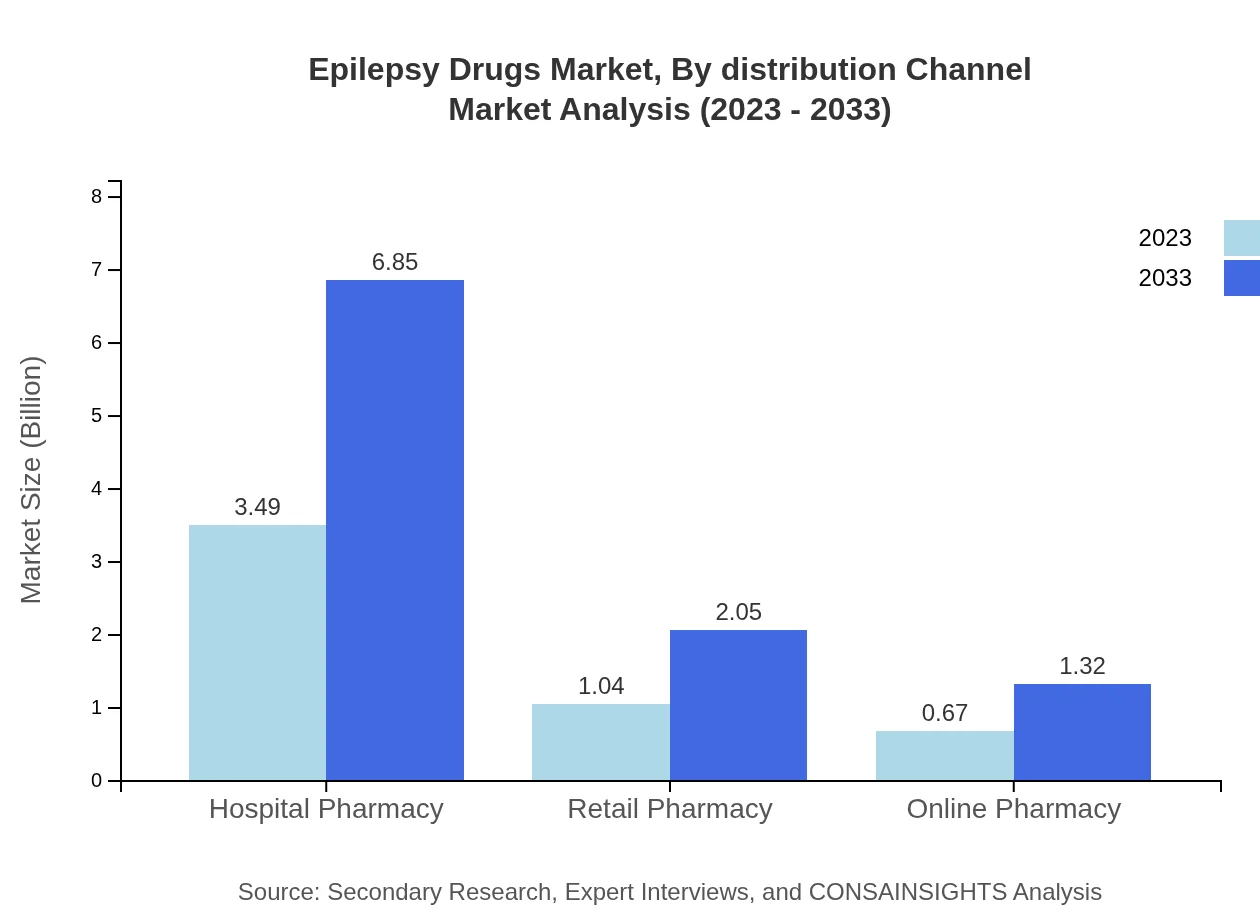
Epilepsy Drugs Market Analysis By Distribution Channel
Hospital pharmacies are central in distributing epilepsy drugs, expected to rise from $3.49 billion in 2023 to $6.85 billion by 2033, holding a market share of 67.07%. Retail pharmacies will grow concurrently from $1.04 billion to $2.05 billion (20.02% share), while online pharmacies will increase from $0.67 billion to $1.32 billion (12.91% share).
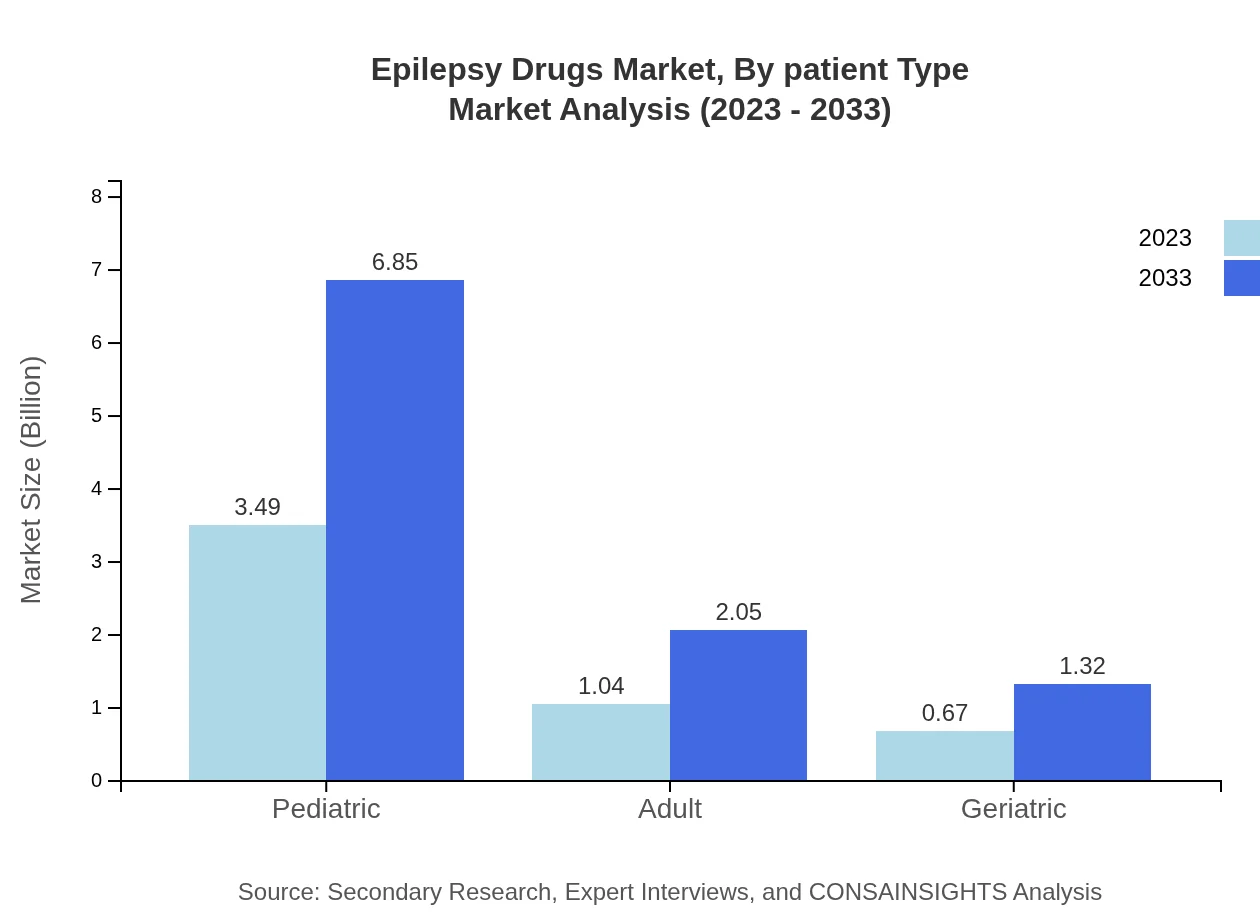
Epilepsy Drugs Market Analysis By Patient Type
Segmenting by patient type, the pediatric category is the largest, expected to grow from $3.49 billion to $6.85 billion (67.07% share). Adults will grow from $1.04 billion to $2.05 billion (20.02%), while geriatric patients' segment will expand from $0.67 billion to $1.32 billion (12.91%).
Epilepsy Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Epilepsy Drugs Industry
Pfizer Inc.:
Pfizer is a global leader in the pharmaceutical industry, known for its extensive portfolio of antiepileptic drugs, including top-tier medications that enhance treatment efficacy for epilepsy patients.UCB S.A.:
UCB is a biopharmaceutical company recognized for its innovation in epilepsy treatment, focusing on developing newer drugs that target various types of seizures.Eisai Co., Ltd.:
Eisai is a prominent player specializing in epilepsy medications, with a commitment to addressing the needs of patients through research and development of effective therapies.Novartis AG:
Novartis is a multinational healthcare company that provides advanced solutions for epilepsy management through its comprehensive range of antiepileptic drugs.Sanofi S.A.:
Sanofi is a leading biopharmaceutical company that develops treatments for various health concerns, including a significant focus on epilepsy drugs to improve patient outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of epilepsy Drugs?
The global epilepsy drugs market was valued at approximately $5.2 billion in 2023 and is projected to grow at a CAGR of 6.8%, indicating significant growth potential in the coming years.
What are the key market players or companies in this epilepsy Drugs industry?
Key players in the epilepsy drugs market include major pharmaceutical companies and biotechnology firms specializing in neurological treatments. Their investments in research and development facilitate innovations in antiepileptic medications.
What are the primary factors driving the growth in the epilepsy drugs industry?
Growth in the epilepsy drugs market is primarily driven by increasing prevalence of epilepsy worldwide, advancements in drug formulations, and heightened awareness and diagnosis of the condition, leading to better treatment outcomes.
Which region is the fastest Growing in the epilepsy drugs market?
The Asia Pacific region is experiencing significant growth in the epilepsy drugs market, with a projected increase from $0.94 billion in 2023 to $1.85 billion by 2033, reflecting a growing patient population and improving healthcare access.
Does ConsaInsights provide customized market report data for the epilepsy Drugs industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the epilepsy drugs industry, ensuring that clients can access relevant insights and analysis for strategic decision-making.
What deliverables can I expect from this epilepsy Drugs market research project?
From the epilepsy drugs market research project, you can expect detailed market analysis reports, segment data insights, company profiles, trends analysis, and tailored recommendations for market entry or expansion.
What are the market trends of epilepsy Drugs?
Market trends in the epilepsy drugs industry include the rising adoption of next-generation antiepileptic drugs, increasing demand for personalized medicine, and a shift towards online pharmacies for greater accessibility.