Epilepsy Market Report
Published Date: 31 January 2026 | Report Code: epilepsy
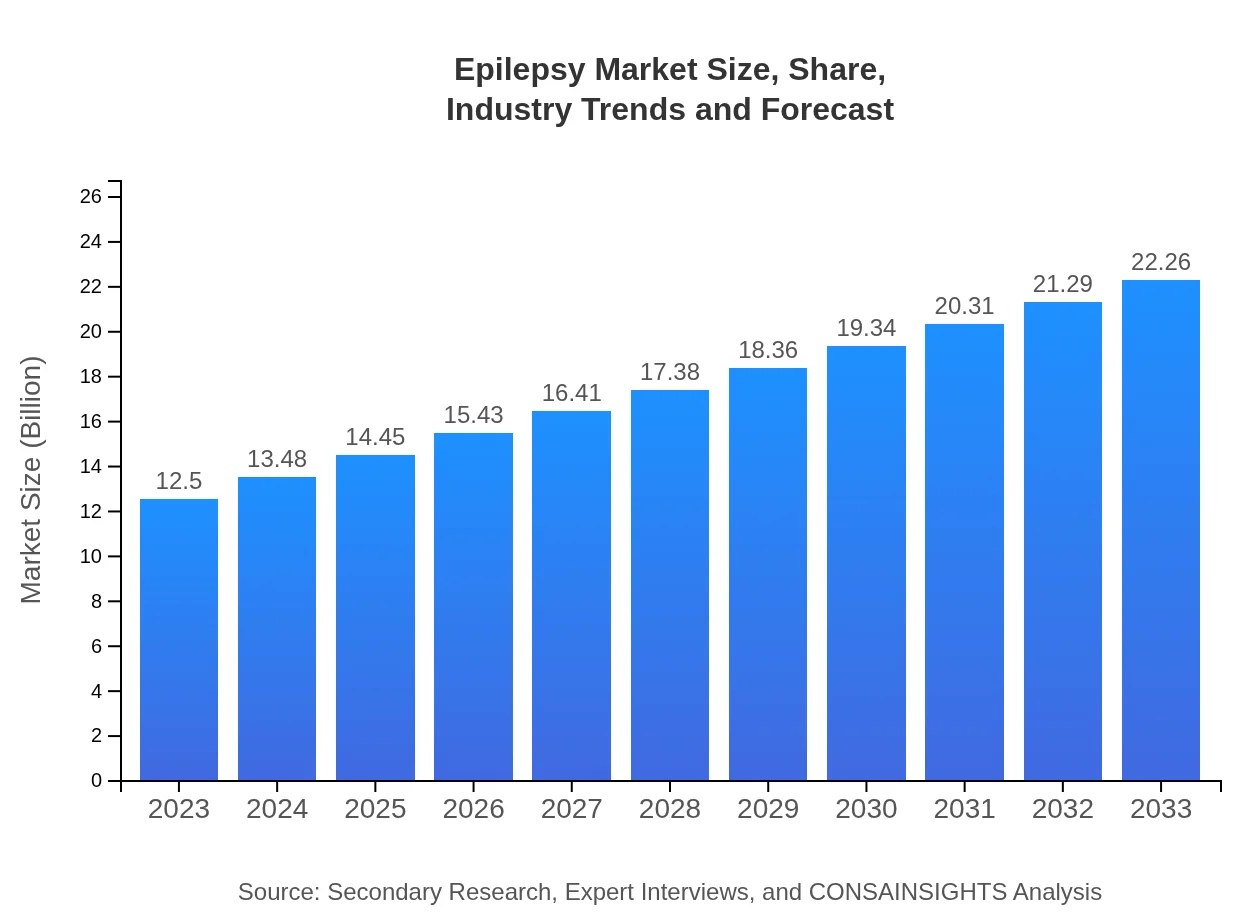
Epilepsy Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the epilepsy market from 2023 to 2033, including insights into market size, growth potential, regional dynamics, key players, and future trends impacting the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $12.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $22.26 Billion |
| Top Companies | Pfizer Inc., Eisai Co., Ltd., UCB SA, AbbVie Inc. |
| Last Modified Date | 31 January 2026 |
Epilepsy Market Overview
Customize Epilepsy Market Report market research report
- ✔ Get in-depth analysis of Epilepsy market size, growth, and forecasts.
- ✔ Understand Epilepsy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Epilepsy
What is the Market Size & CAGR of Epilepsy market in 2023?
Epilepsy Industry Analysis
Epilepsy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Epilepsy Market Analysis Report by Region
Europe Epilepsy Market Report:
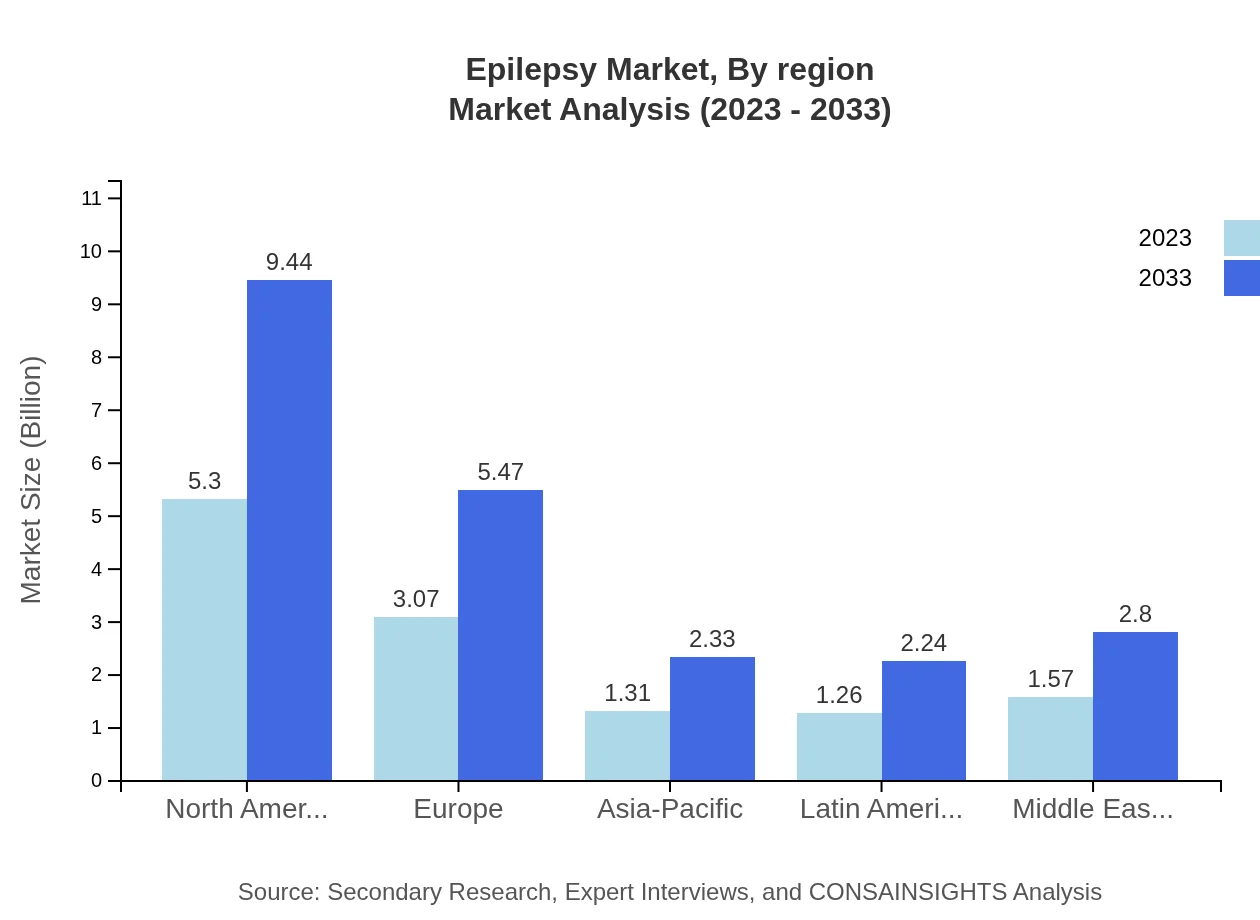
The European market for epilepsy is forecasted to expand from $4.49 billion in 2023 to $7.99 billion by 2033, reflecting a compound growth driven mainly by healthcare reforms and technological innovations in treatment protocols that enhance patient outcomes.Asia Pacific Epilepsy Market Report:
The Asia Pacific region is projected to grow from $2.07 billion in 2023 to $3.69 billion by 2033, driven by increasing healthcare expenditure, advancements in medical technology, and a growing patient population. The rising awareness and availability of treatment options in emerging economies contribute to an optimistic growth outlook.North America Epilepsy Market Report:
North America holds a significant share of the epilepsy market, with a size anticipated to grow from $4.11 billion in 2023 to $7.32 billion by 2033. The high prevalence of epilepsy, coupled with robust healthcare systems and ongoing research activities, positions this region as a leader in market advancements. Current trends indicate a focus on personalized and precision medicine approaches.South America Epilepsy Market Report:
In South America, the market size is expected to increase from $0.89 billion in 2023 to $1.58 billion by 2033. The growth is attributed to increased investments in healthcare infrastructure, though challenges remain in terms of access to care and affordability in some areas.Middle East & Africa Epilepsy Market Report:
The Middle East and Africa's epilepsy market is projected to grow from $0.95 billion in 2023 to $1.69 billion by 2033. Rising awareness of neurological disorders, coupled with efforts to improve healthcare access, underpins this growth, although socioeconomic challenges persist.Tell us your focus area and get a customized research report.
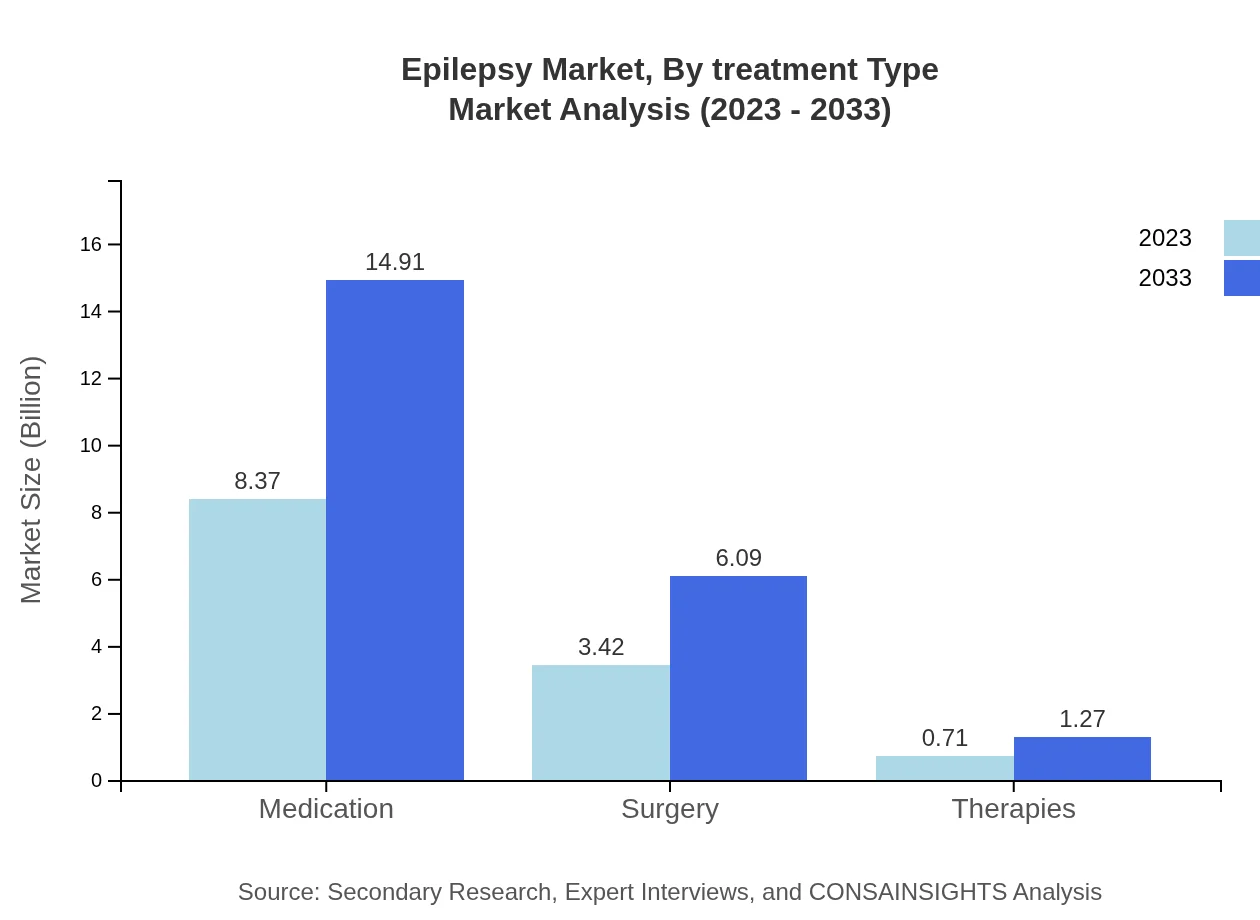
Epilepsy Market Analysis By Treatment Type
The treatment type segment of the epilepsy market includes medication, surgery, and therapies. In 2023, medications are estimated to dominate the market with significant share due to the wide availability of anticonvulsants used across various demographics. Surgery offers a specialized approach for patients who do not respond to medications, while therapies grow in importance as adjunct treatment options.
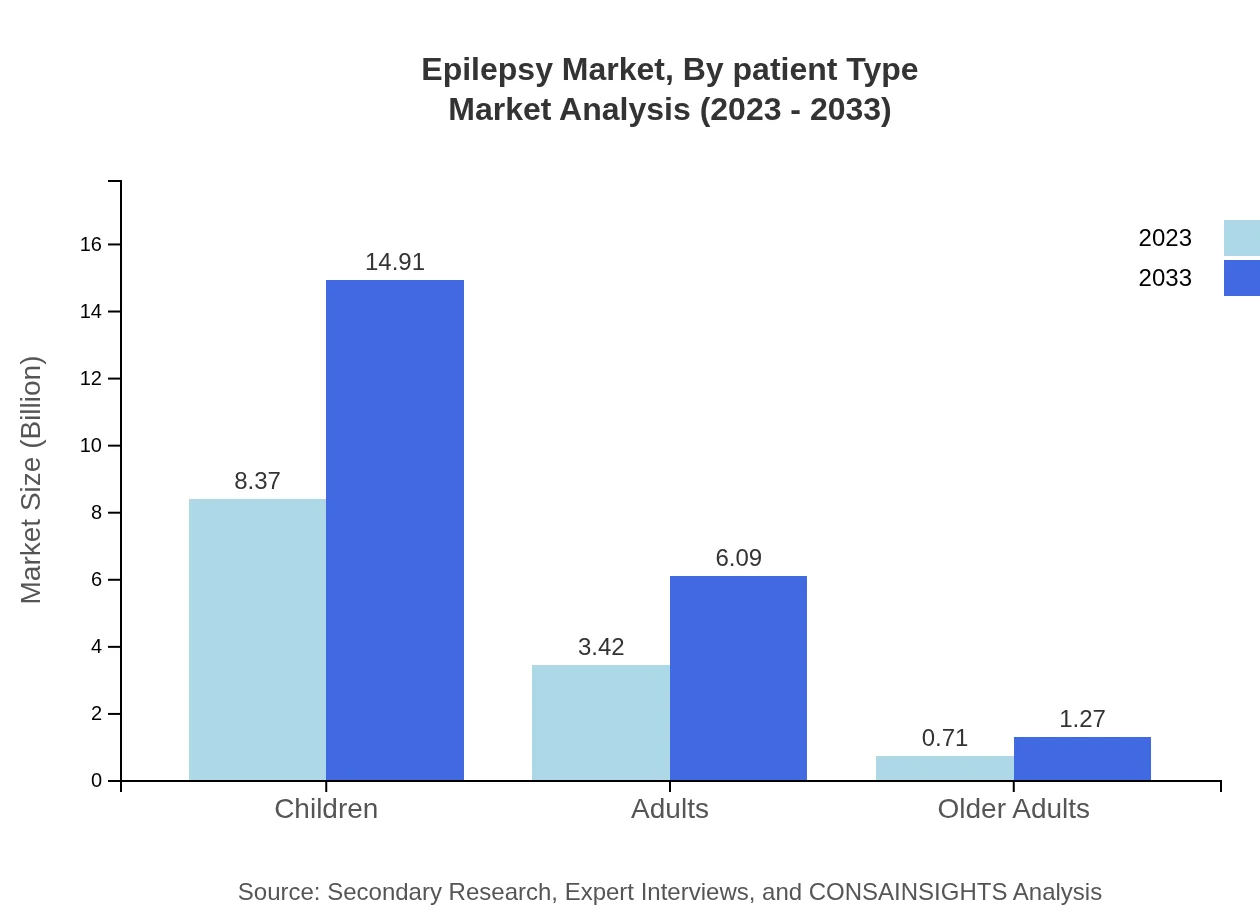
Epilepsy Market Analysis By Patient Type
The breakdown by patient type indicates that children represent a major segment, reflecting the need for pediatric-focused treatment solutions. Nevertheless, the adult population also demands attention due to the varying seizure types and comorbid conditions associated with adulthood.
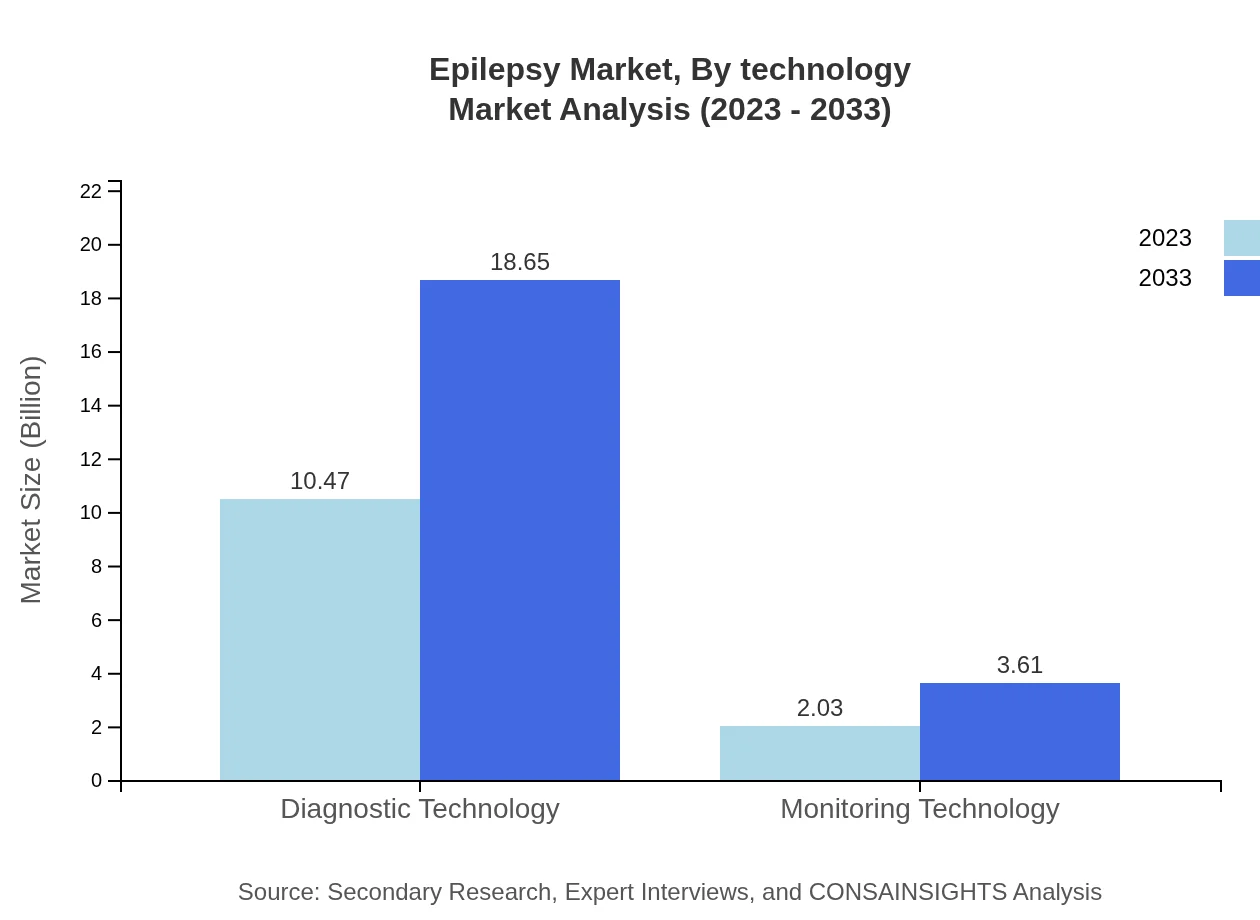
Epilepsy Market Analysis By Technology
Technology plays a crucial role in the epilepsy market, focusing on diagnostics, monitoring, and treatment technologies. Diagnostic technologies lead with the highest market share, leveraging innovative methodologies to enhance seizure detection and management accuracy.
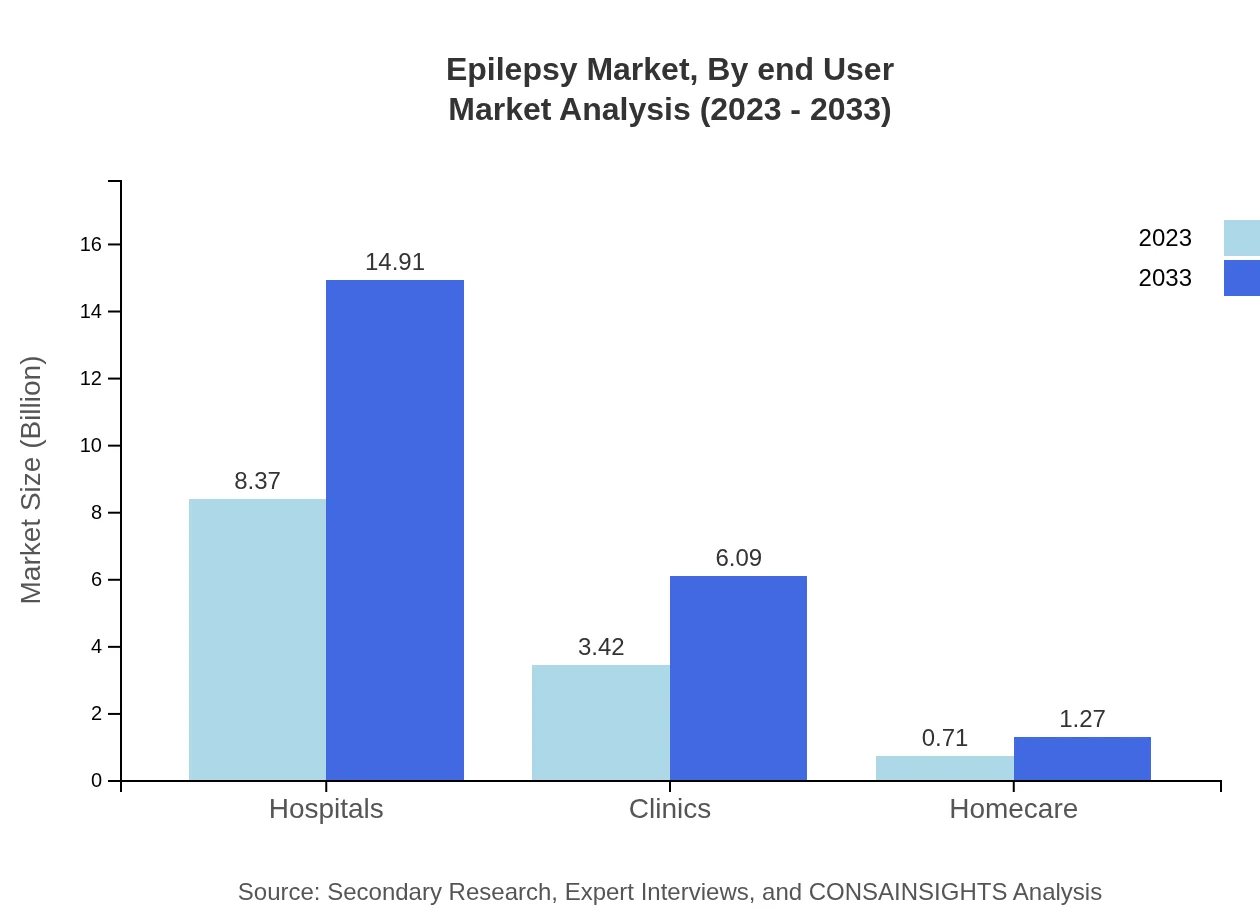
Epilepsy Market Analysis By End User
Hospitals command a significant share of the market in terms of end-user, attributed to their comprehensive treatment capabilities. Clinics and homecare environments emerge as essential components in patient management, with growing acceptance of outpatient care models leading to market expansion.
Epilepsy Market Analysis By Region
Regional analysis reveals North America leading the market in terms of size and innovation, followed by Europe and emerging markets in Asia Pacific witnessing rapid growth due to enhanced healthcare systems.
Epilepsy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Epilepsy Industry
Pfizer Inc.:
A leading biopharmaceutical company, Pfizer focuses on innovative therapies for epilepsy, enhancing patient quality of life through its extensive portfolio.Eisai Co., Ltd.:
A pioneer in the epilepsy sector, Eisai develops Anti-Epileptic Drugs (AEDs) that cater to diverse patient populations, playing a significant role in improving treatment adherence.UCB SA:
UCB specializes in the development of treatments for epilepsy, emphasizing targeted therapies that address unmet medical needs in patients with complex seizure disorders.AbbVie Inc.:
AbbVie is recognized for its commitment to epilepsy treatment development, emphasizing research and development to offer novel therapeutic options.We're grateful to work with incredible clients.









FAQs
What is the market size of epilepsy?
The epilepsy market is valued at approximately $12.5 billion in 2023, with a projected CAGR of 5.8% expected through 2033. This growth reflects increasing awareness and the development of new treatments and technologies in epilepsy management.
What are the key market players or companies in this epilepsy industry?
Key players in the epilepsy market include leading pharmaceutical companies and medical device manufacturers such as UCB, Pfizer, GlaxoSmithKline, and Johnson & Johnson, which are driving innovation and improving patient care through advanced therapeutic solutions.
What are the primary factors driving the growth in the epilepsy industry?
The growth in the epilepsy market is primarily driven by factors such as rising prevalence of epilepsy cases globally, advancements in pharmaceuticals and therapies, increasing research initiatives, and growing healthcare investments to improve treatment accessibility.
Which region is the fastest Growing in the epilepsy market?
The fastest-growing region in the epilepsy market from 2023 to 2033 is Europe, projected to reach $7.99 billion by 2033, reflecting substantial investments in healthcare infrastructure and increasing incidence of epilepsy.
Does ConsaInsights provide customized market report data for the epilepsy industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the epilepsy industry. This includes detailed insights on market trends, competitive analysis, and regional forecasts to support strategic decision-making.
What deliverables can I expect from this epilepsy market research project?
From the epilepsy market research project, you can expect comprehensive deliverables including market size analysis, growth forecasts, segmentation breakdown, competitive landscape reports, and strategic recommendations based on current market dynamics.
What are the market trends of epilepsy?
Current market trends in the epilepsy sector include increased focus on personalized medicine, growth of telehealth services, a rise in innovative monitoring technologies, and enhanced collaboration between pharmaceutical companies and healthcare providers to improve patient outcomes.