Epinephrine Autoinjector Market Report
Published Date: 31 January 2026 | Report Code: epinephrine-autoinjector
Epinephrine Autoinjector Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Epinephrine Autoinjector market, covering insights into market size, trends, and forecasts from 2023 to 2033. It highlights key segments, regional developments, and industry players shaping the future of this critical medical device market.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
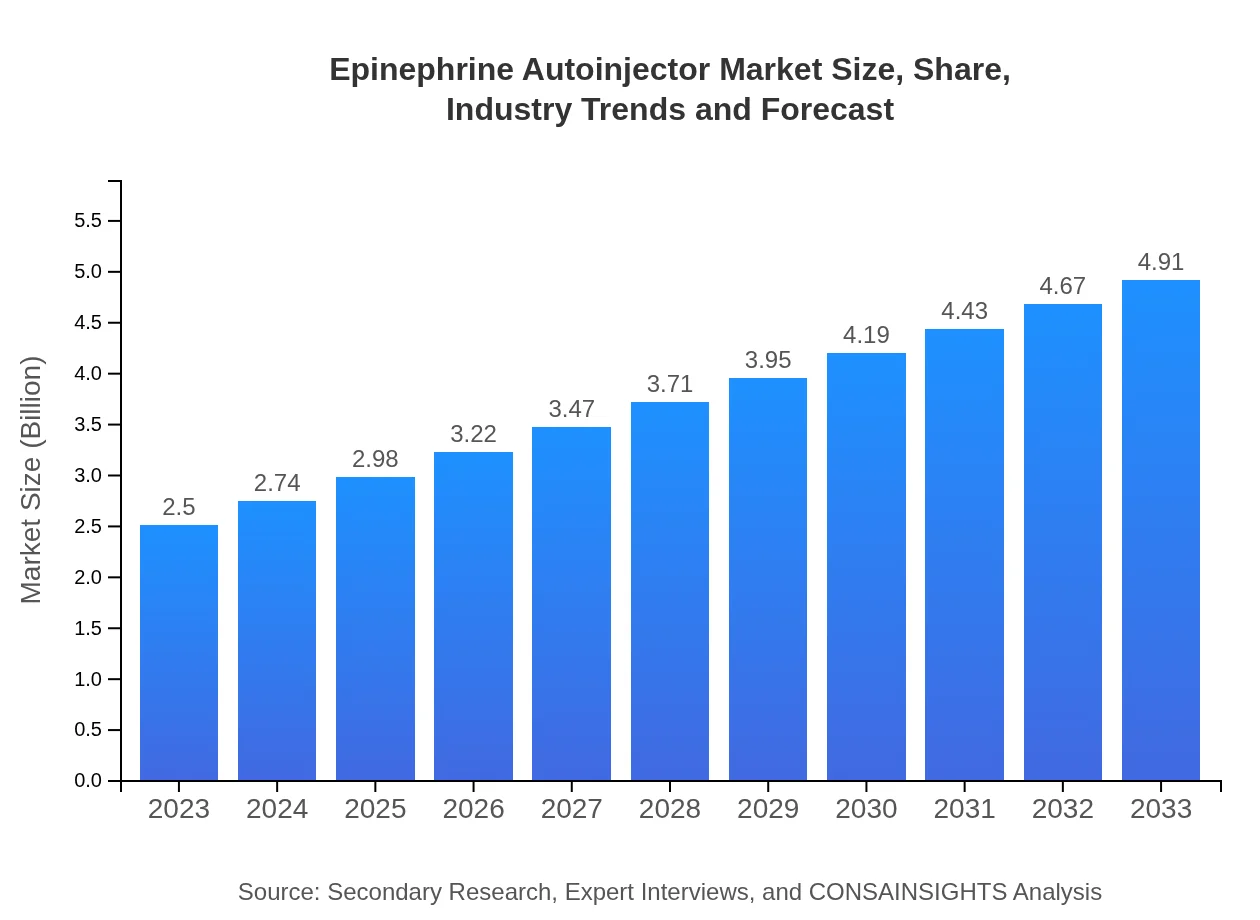
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Mylan N.V., Sanofi, Teva Pharmaceutical Industries Ltd., Eli Lilly and Company |
| Last Modified Date | 31 January 2026 |
Epinephrine Autoinjector Market Overview
Customize Epinephrine Autoinjector Market Report market research report
- ✔ Get in-depth analysis of Epinephrine Autoinjector market size, growth, and forecasts.
- ✔ Understand Epinephrine Autoinjector's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Epinephrine Autoinjector
What is the Market Size & CAGR of Epinephrine Autoinjector market in 2023?
Epinephrine Autoinjector Industry Analysis
Epinephrine Autoinjector Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Epinephrine Autoinjector Market Analysis Report by Region
Europe Epinephrine Autoinjector Market Report:
The European market is also witnessing significant growth, with the market size expected to increase from $0.66 billion in 2023 to $1.30 billion by 2033. This growth is attributed to the supportive regulatory environment and increasing public awareness of allergic disorders.Asia Pacific Epinephrine Autoinjector Market Report:
The Asia Pacific region shows promising growth due to increasing awareness about allergy management and substantial investments in healthcare infrastructure. The market size is projected to grow from $0.48 billion in 2023 to $0.95 billion by 2033, driven by rising disposable incomes and a growing prevalence of allergic conditions.North America Epinephrine Autoinjector Market Report:
North America dominates the Epinephrine Autoinjector market, with a valuation of $0.87 billion in 2023 rising to $1.71 billion by 2033. Strong demand is propelled by advanced healthcare systems, high allergy prevalence, and direct-to-consumer marketing strategies adopted by major players.South America Epinephrine Autoinjector Market Report:
In South America, the market is gaining traction, with a current value of $0.25 billion projected to increase to $0.49 billion by 2033. The rising incidence of allergies, coupled with improved healthcare access and increased government initiatives, contributes to market development in this region.Middle East & Africa Epinephrine Autoinjector Market Report:
In the Middle East and Africa, the market is projected to grow from $0.24 billion in 2023 to $0.47 billion by 2033. Factors contributing to this growth include rising healthcare expenditures and an increased focus on treating anaphylaxis due to a growing allergic population.Tell us your focus area and get a customized research report.
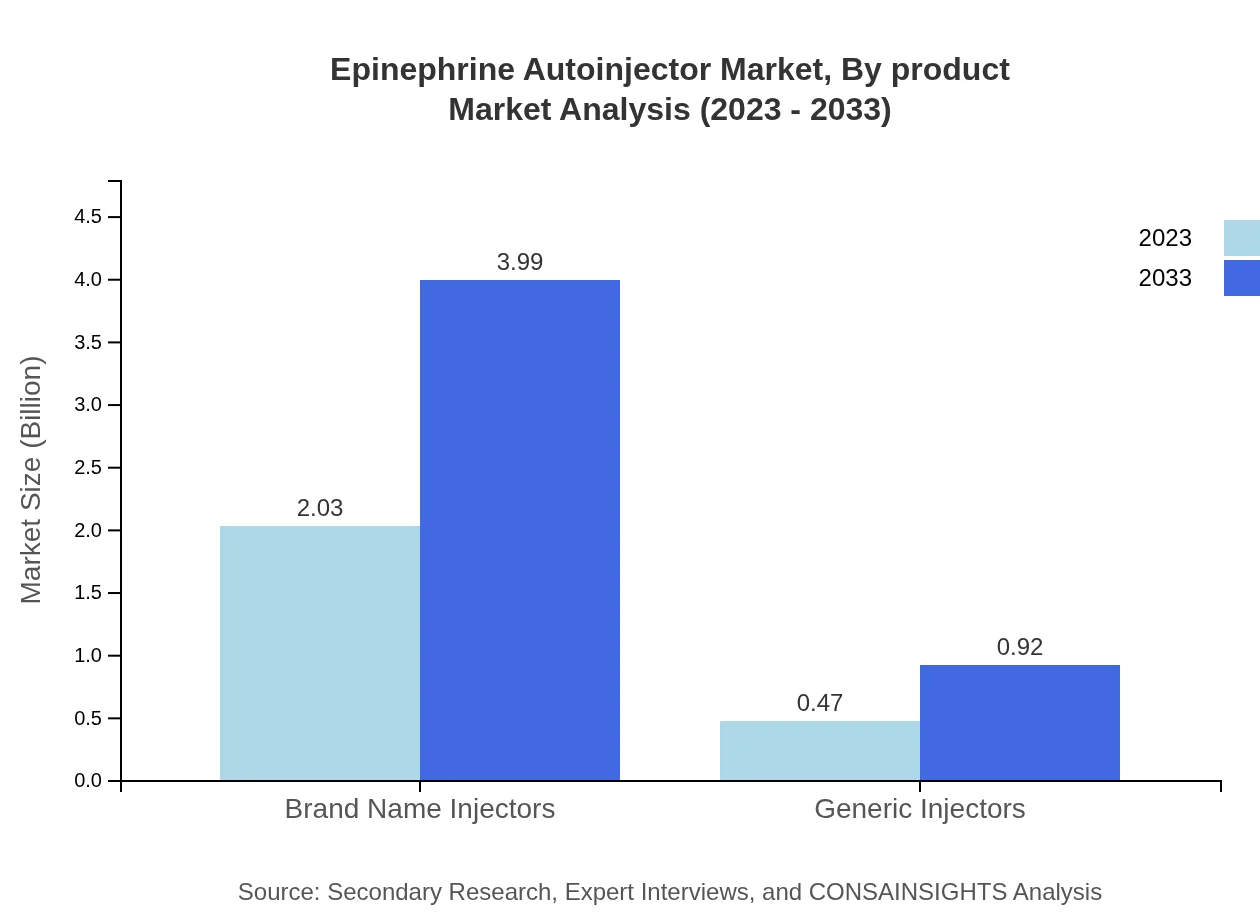
Epinephrine Autoinjector Market Analysis By Product
The Epinephrine Autoinjector market is primarily segmented into brand name injectors and generic injectors. Brand name injectors dominate the market with a size of $2.03 billion in 2023, expected to rise to $3.99 billion by 2033, comprising a steady market share of 81.18%. Conversely, generic injectors are growing as affordability becomes a key concern, projected to grow from $0.47 billion to $0.92 billion. This dual presence of brand and generic products highlights competition and diversity within the market.
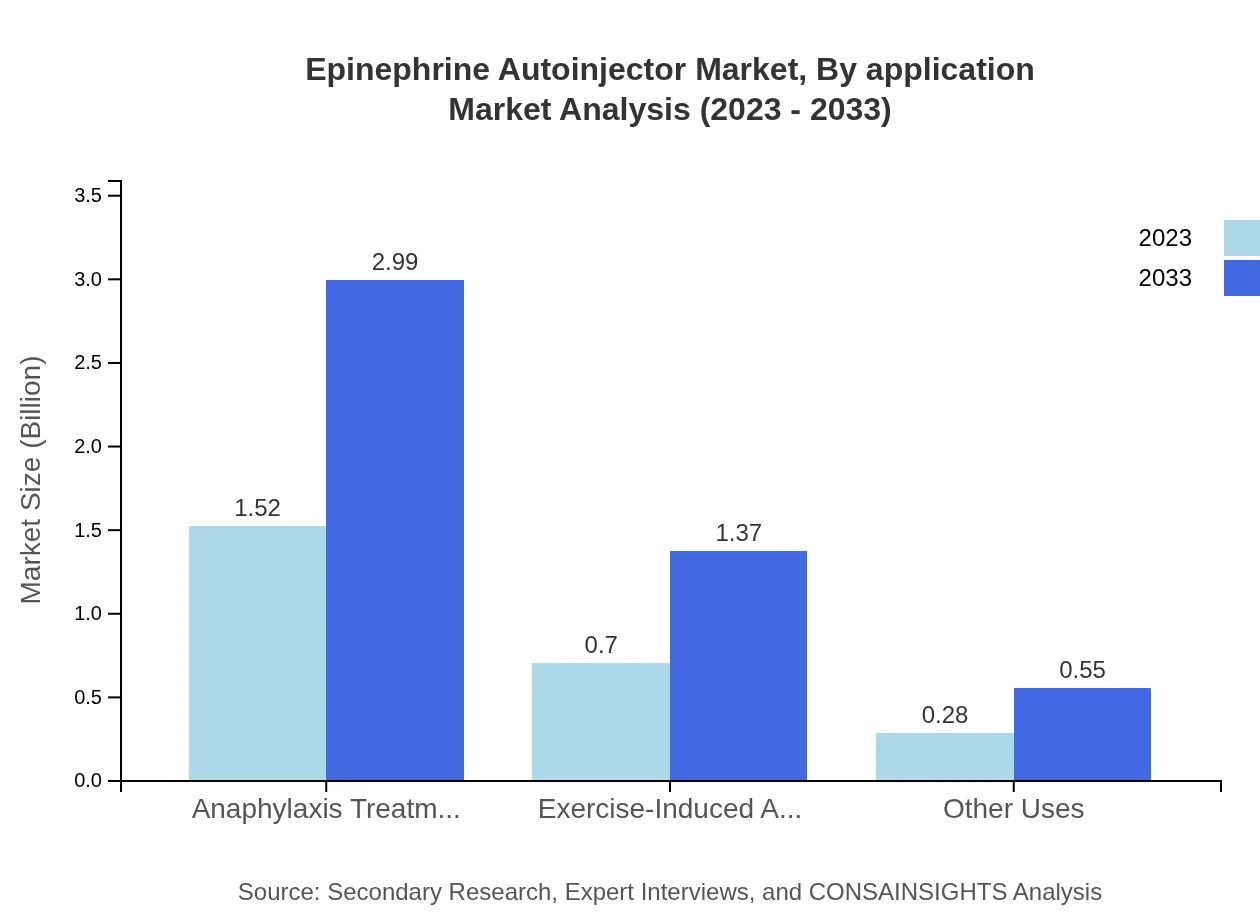
Epinephrine Autoinjector Market Analysis By Application
The market is segmented into applications such as anaphylaxis treatment, exercise-induced anaphylaxis, and other utilizations. Anaphylaxis treatment holds a substantial market share of 60.83% and is expected to expand from $1.52 billion in 2023 to $2.99 billion by 2033. Exercise-induced anaphylaxis applications currently represent a market of $0.70 billion, anticipated to reach $1.37 billion, highlighting growing awareness and diagnosis of exercise-related allergies.
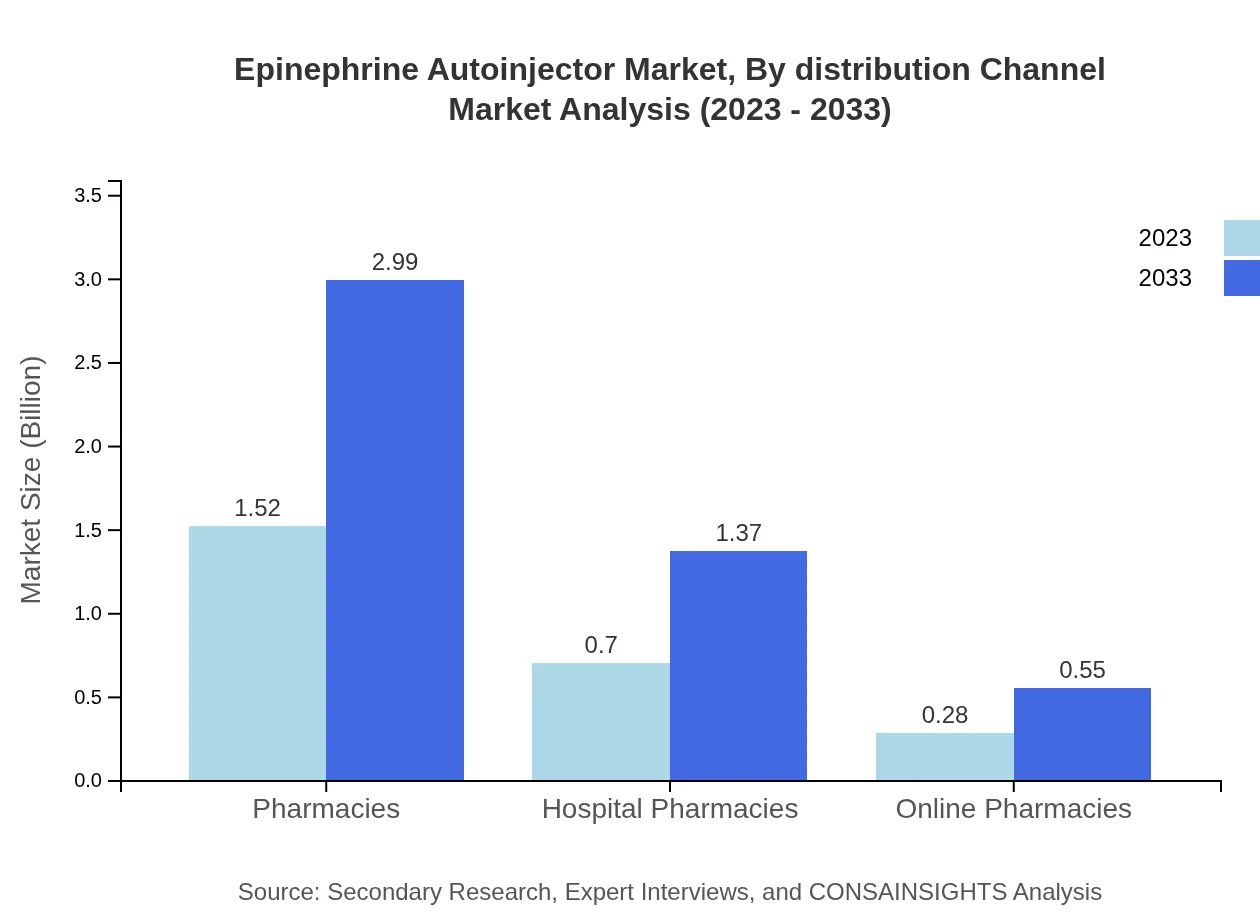
Epinephrine Autoinjector Market Analysis By Distribution Channel
Distribution channels play a significant role in market reach. Pharmacies are the primary channel, expected to maintain a market size of $1.52 billion in 2023 and rise to $2.99 billion by 2033, representing over 60% of market share. Online pharmacies are gaining traction, promoting convenience and accessibility, projected to grow from $0.28 billion to $0.55 billion over the same period, catering to tech-savvy consumers.
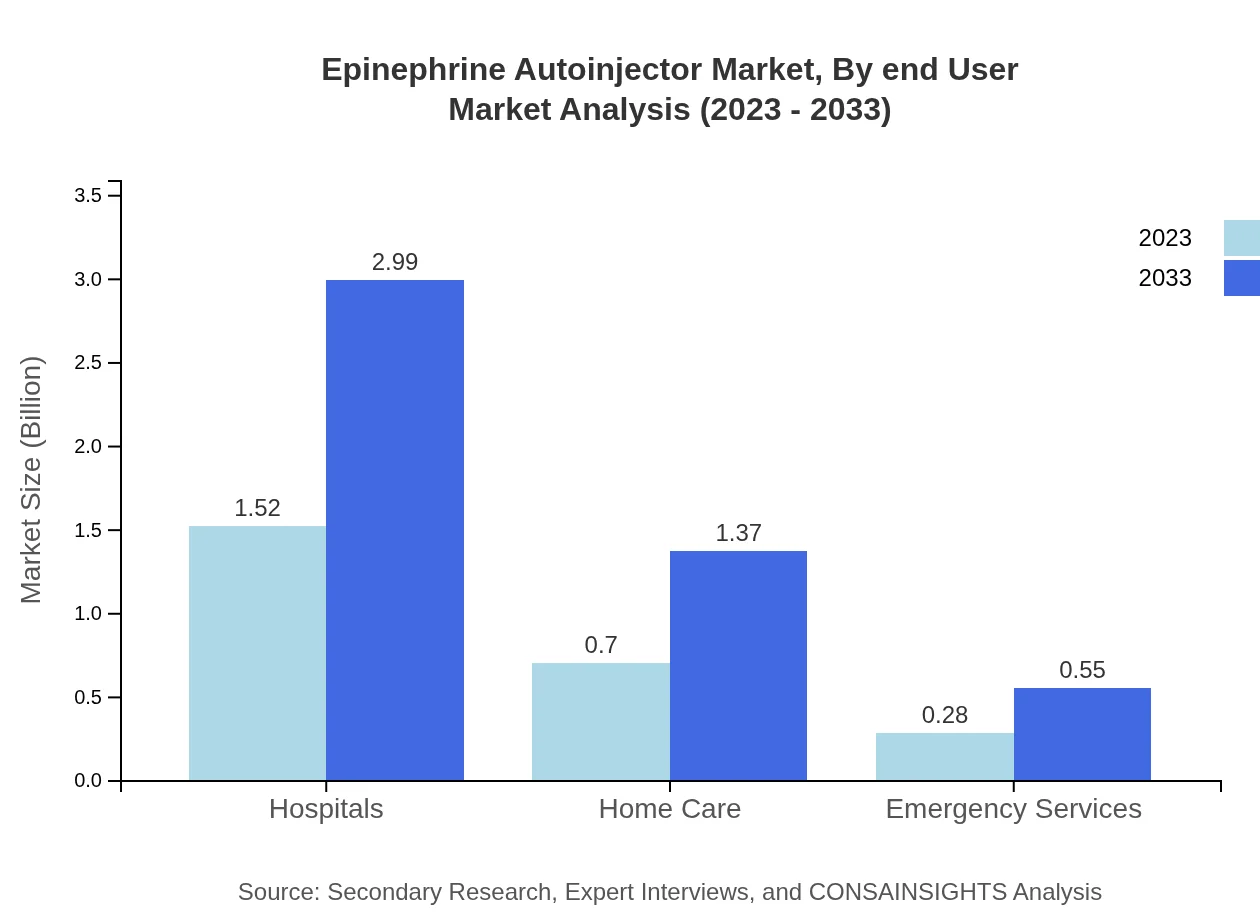
Epinephrine Autoinjector Market Analysis By End User
End-user segmentation reveals substantial demands from hospitals, representing $1.52 billion in 2023, increasing to $2.99 billion by 2033, showcasing 60.83% market share. Home care services are emerging as a key segment, projected to double in size, reflecting changing preferences towards at-home management of allergic conditions. Emergency services, although smaller at $0.28 billion, show growth potential, particularly in emergency interventions.
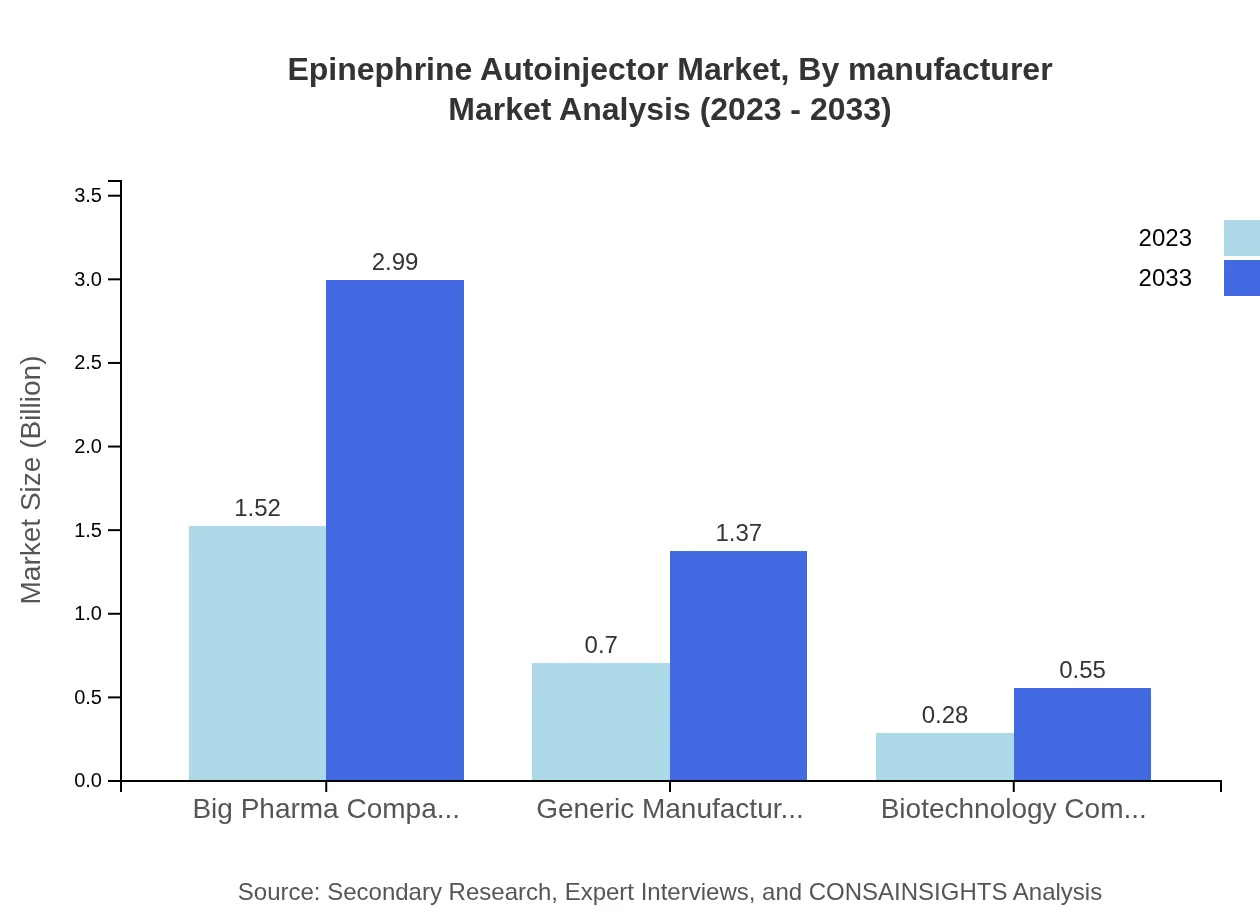
Epinephrine Autoinjector Market Analysis By Manufacturer
The market is dominated by big pharmaceutical companies, contributing a sizable market of $1.52 billion in 2023 and expected to grow to $2.99 billion by 2033, representing 60.83% share. Generic manufacturers are also significant players, catering to cost-sensitive consumers projected to grow from $0.70 billion to $1.37 billion, emphasizing a balancing act between brand loyalty and affordability.
Epinephrine Autoinjector Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Epinephrine Autoinjector Industry
Mylan N.V.:
Mylan is a leading producer of generic and specialty pharmaceuticals. Their EpiPen product has become synonymous with allergy treatment, reflecting strong market presence and brand recognition.Sanofi:
Sanofi is a global healthcare leader, known for its innovative drugs and vaccines. The company’s Auvi-Q device showcases advanced technology and design, focusing on user-friendly features for emergency situations.Teva Pharmaceutical Industries Ltd.:
Teva is one of the largest generic pharmaceutical companies in the world, producing affordable options that expand access to life-saving medications like epinephrine autoinjectors.Eli Lilly and Company:
Eli Lilly is prominent in the biopharmaceutical industry and has been involved in the development of epinephrine products, providing options to pediatric and adult patients with severe allergies.We're grateful to work with incredible clients.









FAQs
What is the market size of epinephrine Autoinjectors?
The global market size for epinephrine autoinjectors is projected to reach approximately $2.5 billion by 2033, growing at a robust CAGR of 6.8% from its current valuation in 2023.
What are the key market players or companies in the epinephrine Autoinjector industry?
Key players in the epinephrine autoinjector market include major pharmaceutical companies and generic manufacturers, with significant contributions from big pharma companies and biotechnological firms specializing in allergy medications.
What are the primary factors driving the growth in the epinephrine Autoinjector industry?
Growth in the epinephrine autoinjector market is driven by rising incidence of anaphylaxis, increased awareness of allergy treatments, advancements in auto-injection technology, and growing adoption of self-injection devices among patients.
Which region is the fastest Growing in the epinephrine Autoinjector market?
North America is currently the fastest-growing region, expected to expand from a market size of $0.87 billion in 2023 to $1.71 billion by 2033, due to high prevalence of allergies and strong healthcare infrastructure.
Does ConsaInsights provide customized market report data for the epinephrine Autoinjector industry?
Yes, Consainsights offers customized market report data for the epinephrine autoinjector industry, allowing clients to obtain tailored insights that cater to specific business needs or research queries.
What deliverables can I expect from this epinephrine Autoinjector market research project?
Deliverables from the epinephrine autoinjector market research project include comprehensive market analysis, competitive landscape assessment, segmentation data, and future market forecasts across various regions and segments.
What are the market trends of epinephrine Autoinjectors?
Market trends indicate an increasing shift towards home care usage of epinephrine autoinjectors, with significant growth in both brand name and generic injectors, emphasizing patient autonomy and accessibility in treating allergic reactions.