Etanercept Market Report
Published Date: 31 January 2026 | Report Code: etanercept
Etanercept Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Etanercept market, examining market size, trends, regional performances, and key players. Insights are offered for the forecast period of 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
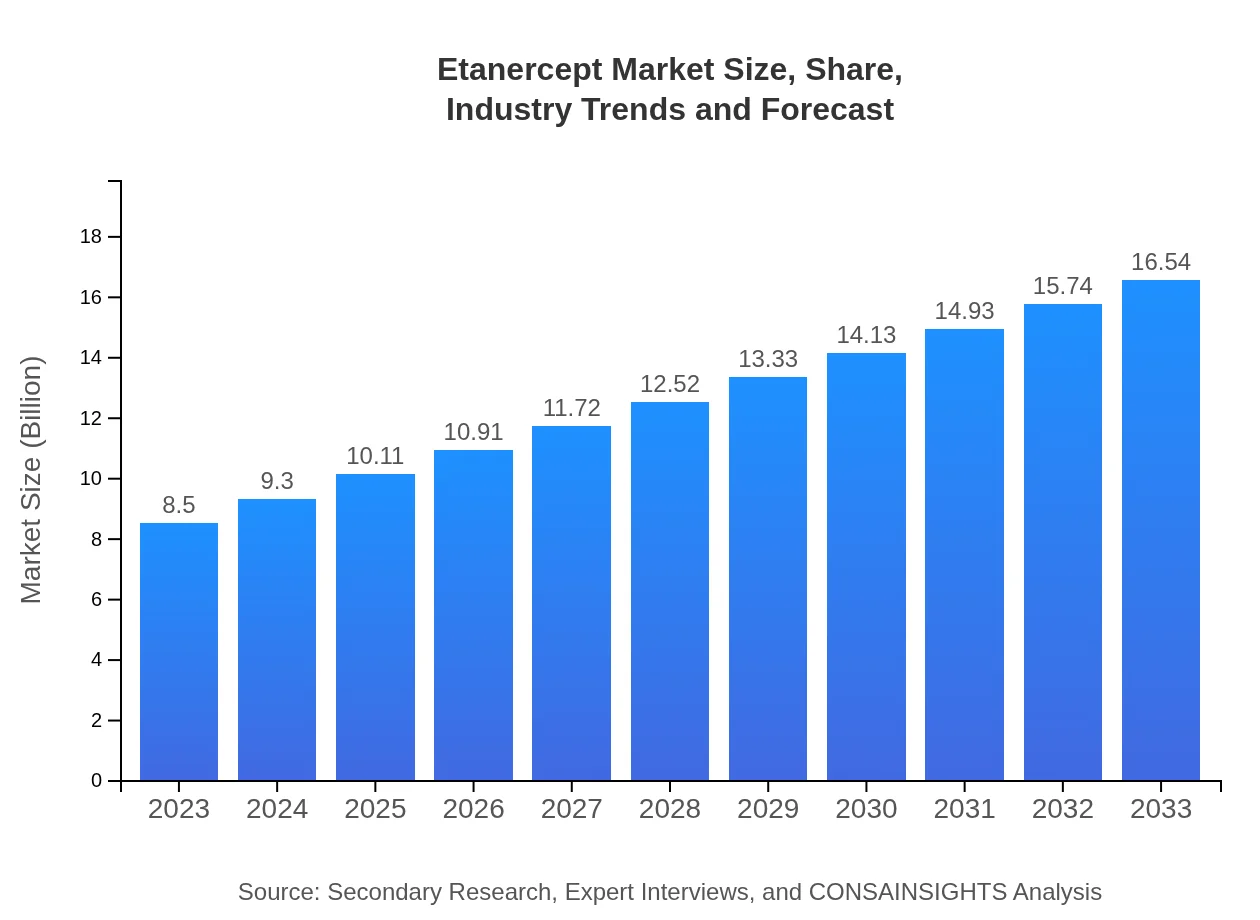
| 2023 Market Size | $8.50 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $16.54 Billion |
| Top Companies | Amgen Inc., Pfizer Inc., Johnson & Johnson, Samsung Biologics |
| Last Modified Date | 31 January 2026 |
Etanercept Market Overview
Customize Etanercept Market Report market research report
- ✔ Get in-depth analysis of Etanercept market size, growth, and forecasts.
- ✔ Understand Etanercept's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Etanercept
What is the Market Size & CAGR of Etanercept market in 2023?
Etanercept Industry Analysis
Etanercept Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Etanercept Market Analysis Report by Region
Europe Etanercept Market Report:
In Europe, the Etanercept market is projected to grow from $2.17 billion in 2023 to $4.23 billion by 2033. The region benefits from strong regulatory approvals and high treatment rates for autoimmune disorders, reflecting robust healthcare strategies. The rise of biosimilars is expected to increase market competitiveness, potentially leading to enhanced patient access.Asia Pacific Etanercept Market Report:
The Asia Pacific region for the Etanercept market showcases a projected growth from $1.65 billion in 2023 to approximately $3.21 billion by 2033. Factors driving growth include increasing healthcare infrastructure, rising prevalence of autoimmune diseases, and improved affordability of treatments. The region also benefits from a growing awareness of biologic therapies among healthcare providers and patients.North America Etanercept Market Report:
North America remains the largest market for Etanercept, with an expected growth from $3.06 billion in 2023 to $5.96 billion by 2033. The high market value is driven by the concentrated demand for advanced therapeutic options, extensive research activities, and favorable reimbursement policies. The implementation of innovative treatment methodologies is also contributing to market expansion.South America Etanercept Market Report:
In South America, the Etanercept market is expected to grow from $0.64 billion in 2023 to $1.24 billion by 2033. This market growth can be attributed to improving healthcare access, rising investment in healthcare systems, and increased patient awareness regarding treatment options. Diverse healthcare policies also aim to enhance the availability of advanced treatments.Middle East & Africa Etanercept Market Report:
The Middle East and Africa region will see growth from $0.98 billion in 2023 to $1.91 billion by 2033. Increasing healthcare investments, efforts to enhance drug availability, and greater healthcare awareness among the population are key factors contributing to the anticipated growth. The introduction of targeted healthcare initiatives is also making treatment options more accessible.Tell us your focus area and get a customized research report.
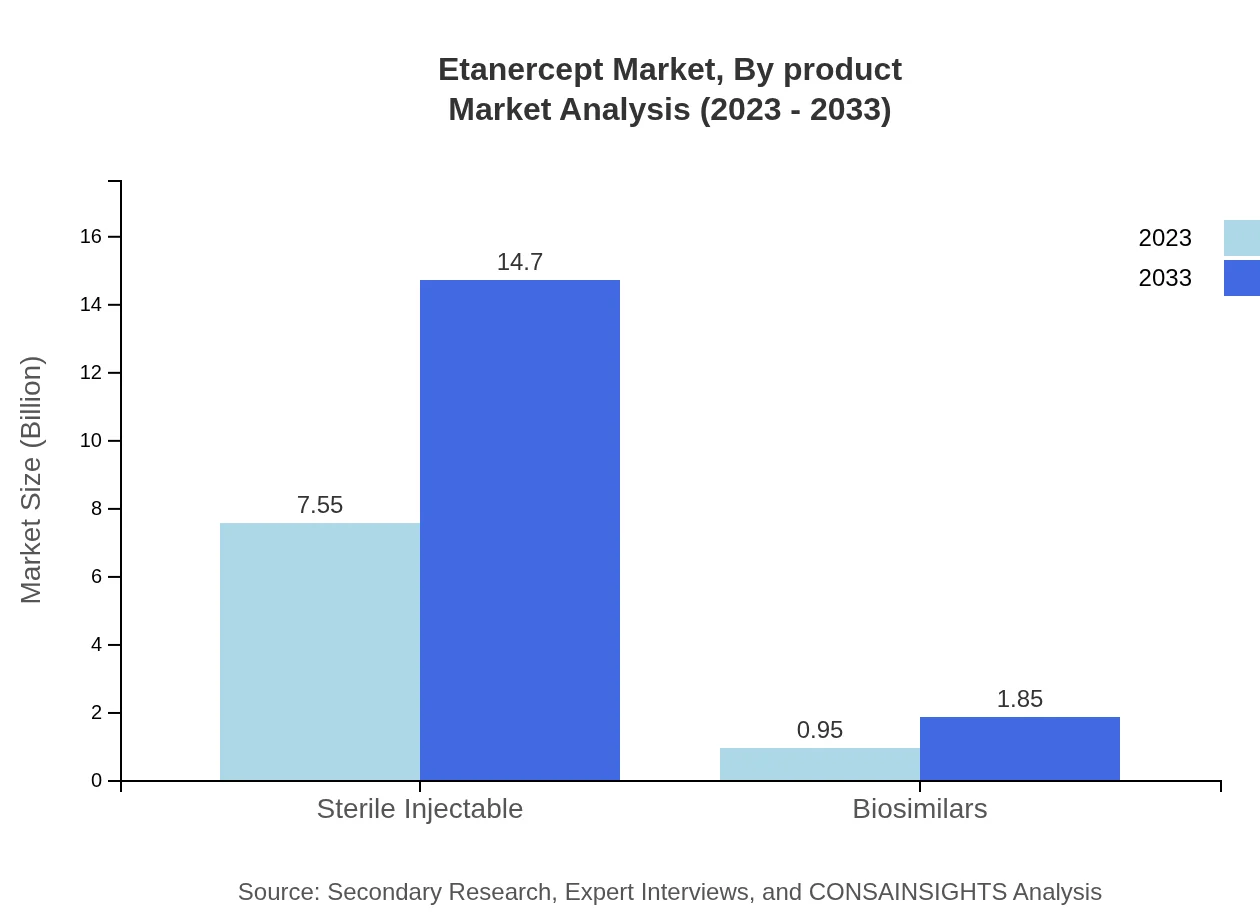
Etanercept Market Analysis By Product
The product segment predominantly features sterile injectables, accounting for significant market share due to their established efficacy and safety profiles. The market for sterile injectables is projected to reach $14.70 billion by 2033. Additionally, the biosimilars segment is expected to grow in popularity, reaching $1.85 billion, reflecting increased patient choice and affordability.
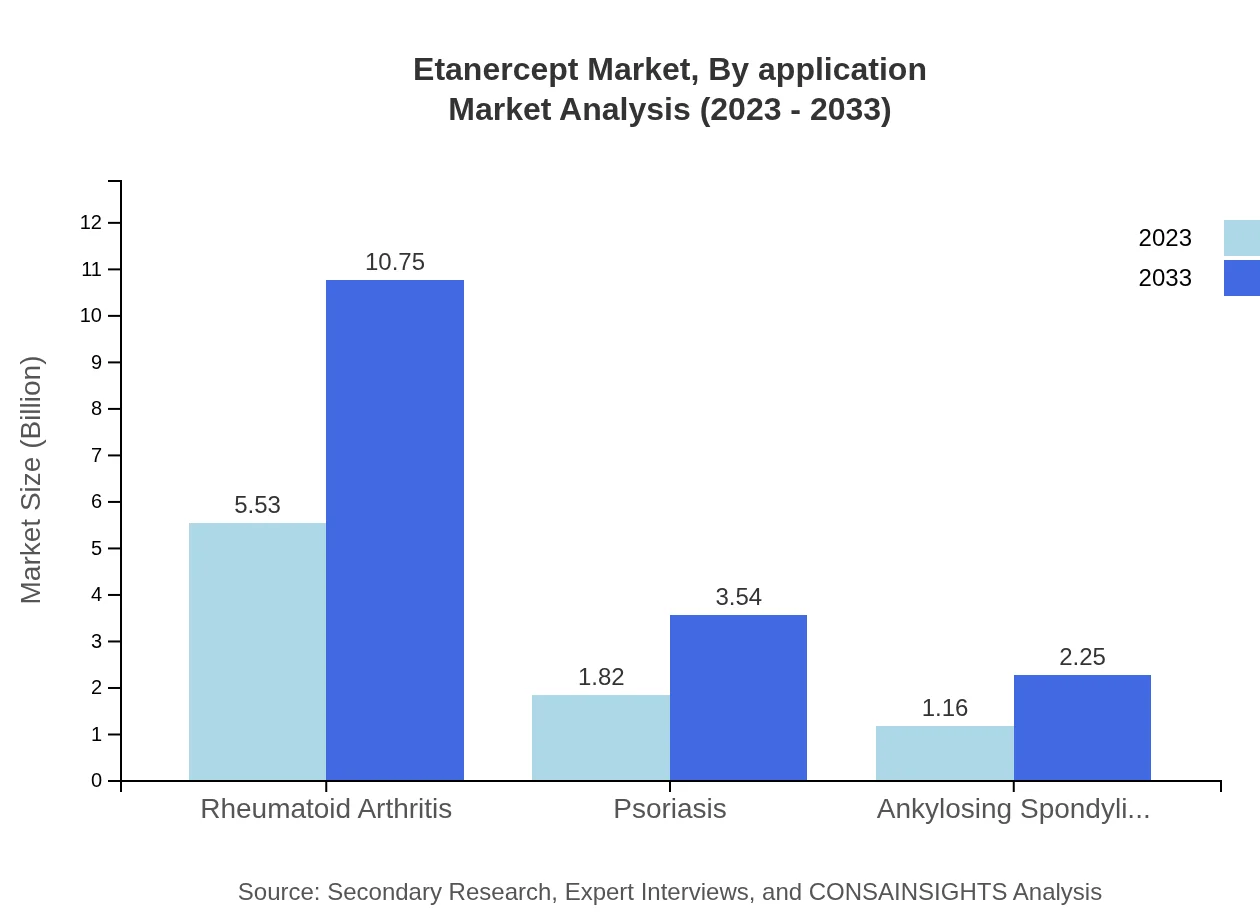
Etanercept Market Analysis By Application
Within the application segment, rheumatoid arthritis demonstrates the largest market share, projected to remain at 65.01% by 2033. Psoriasis and ankylosing spondylitis are also significant contributors, with market sizes expected at $3.54 billion and $2.25 billion respectively, highlighting the importance of Etanercept in treating chronic inflammatory conditions.
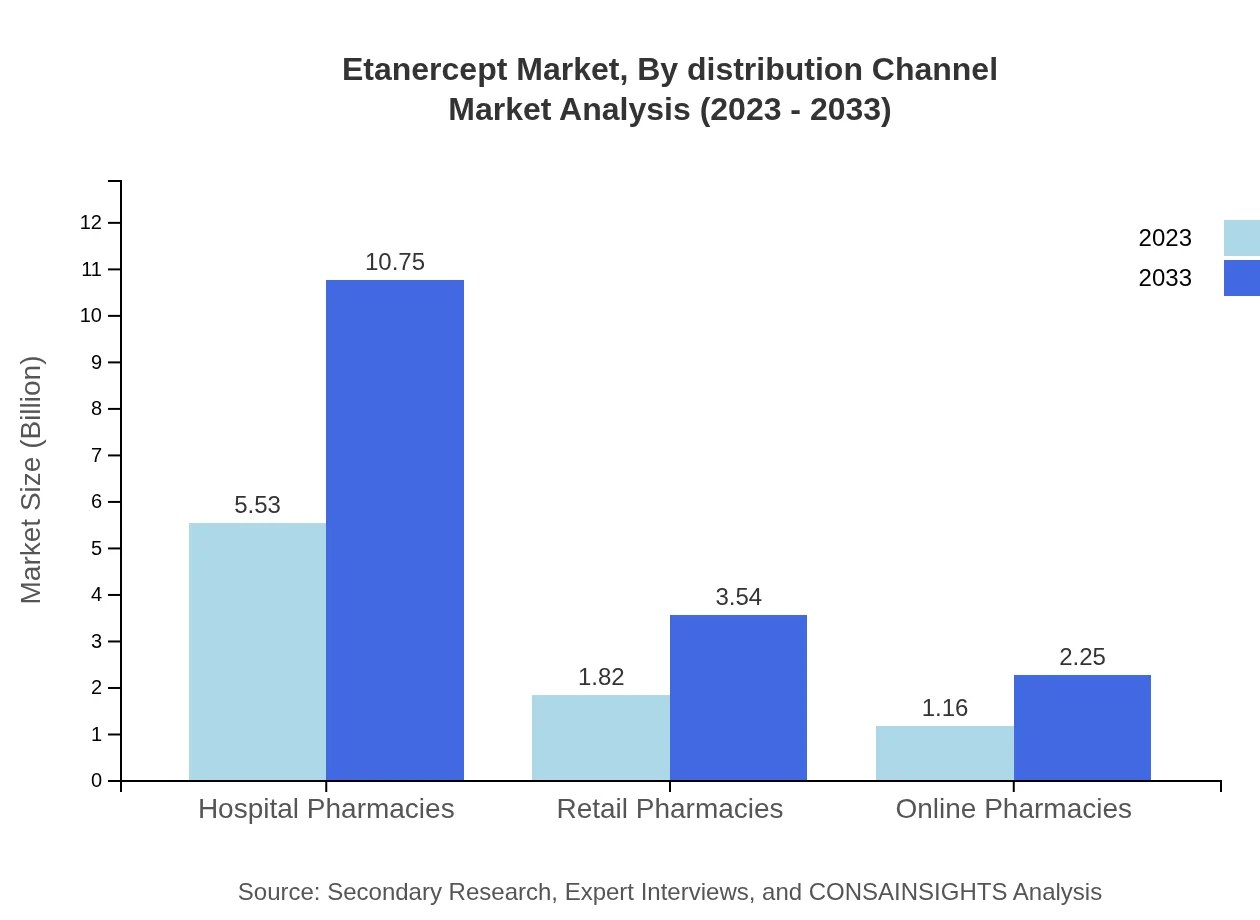
Etanercept Market Analysis By Distribution Channel
Distribution channels consist primarily of hospital pharmacies, making up about 65.01% of market share throughout the forecast period. Retail pharmacies and online pharmacies contribute significantly to accessibility, with market projections of $3.54 billion and $2.25 billion respectively for 2033. This diversified approach ensures that patients can obtain Etanercept through various healthcare settings.
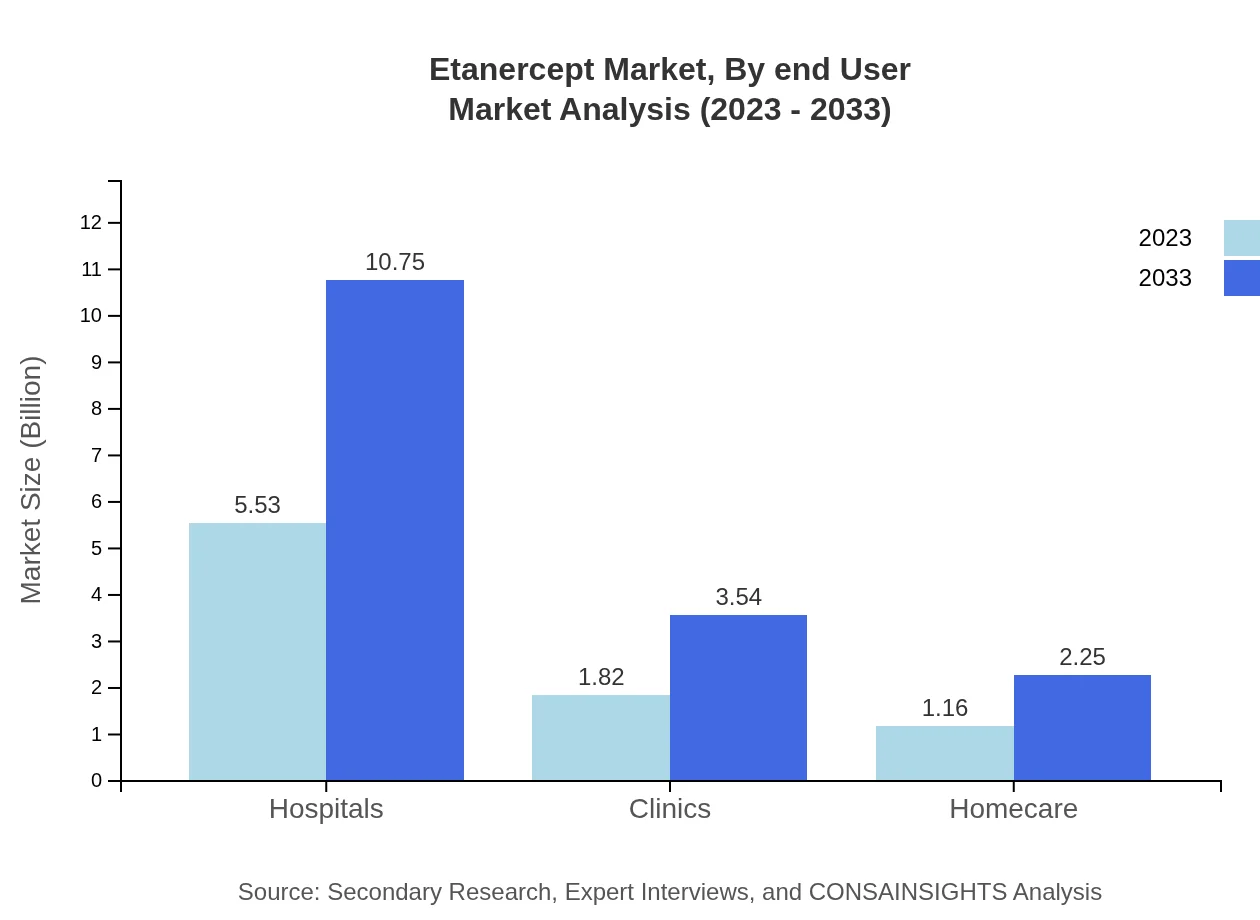
Etanercept Market Analysis By End User
Hospital settings remain the primary end-user for Etanercept, maintaining a market share of 65.01%. Clinics and homecare settings also represent critical domains for administering this treatment, with respective market sizes projected at $3.54 billion and $2.25 billion by 2033, indicating a focused approach to managing patient care across different environments.
Etanercept Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Etanercept Industry
Amgen Inc.:
Amgen is a leading biotechnology firm recognized for its innovations in biologic therapies, including Etanercept. The company's commitment to research and patient care has significantly driven advancements in the treatment landscape for autoimmune diseases.Pfizer Inc.:
Pfizer is a global pharmaceutical leader known for its comprehensive biological treatment portfolio. Through strategic partnerships and a rich pipeline of biosimilars, Pfizer plays a pivotal role in the ongoing evolution of the Etanercept market.Johnson & Johnson:
Johnson & Johnson is a pioneering organization in the pharmaceutical sector, contributing to the development and marketing of advanced therapeutic options in the autoimmune domain, thereby influencing Etanercept's accessibility.Samsung Biologics:
Samsung Biologics is emerging as a key player in the biosimilars market, offering competitive alternatives to Etanercept that aim to increase treatment access and reduce healthcare costs across global markets.We're grateful to work with incredible clients.









FAQs
What is the market size of etanercept?
The global etanercept market is valued at approximately $8.5 billion in 2023, and it is projected to grow at a CAGR of 6.7% through 2033, indicating significant expansion and demand within the industry.
What are the key market players or companies in the etanercept industry?
Key players in the etanercept market include major pharmaceutical companies such as Amgen, Pfizer, and Johnson & Johnson. These firms are recognized for their advancements in biotechnology and their commitment to developing targeted therapies for autoimmune diseases.
What are the primary factors driving the growth in the etanercept industry?
Growth in the etanercept market is driven by the increasing prevalence of autoimmune diseases, advancements in biopharmaceutical technologies, and a growing emphasis on personalized medicine. Additionally, the rising geriatric population contributes significantly to the demand for effective treatments.
Which region is the fastest Growing in the etanercept market?
The Asia Pacific region is the fastest-growing in the etanercept market, with market size projected to increase from $1.65 billion in 2023 to $3.21 billion by 2033, reflecting an accelerated demand for therapeutic solutions in emerging economies.
Does Consainsights provide customized market report data for the etanercept industry?
Yes, Consainsights offers tailored market research reports for the etanercept industry. Clients can expect customized insights that address specific queries, market dynamics, and competitive landscapes, ensuring relevance to their business needs.
What deliverables can I expect from this etanercept market research project?
From the etanercept market research project, clients can expect comprehensive reports detailing market size, growth forecasts, regional analysis, competitive landscape, and trends. These deliverables provide vital information for strategic decision-making.
What are the market trends of etanercept?
Current trends in the etanercept market include the increasing adoption of biosimilars, a shift towards integrated care approaches, and rising investments in research and development. These trends indicate a dynamic market responding to patients' needs.