Extracorporeal Membrane Oxygenation Ecmo Systems Market Report
Published Date: 31 January 2026 | Report Code: extracorporeal-membrane-oxygenation-ecmo-systems
Extracorporeal Membrane Oxygenation Ecmo Systems Market Size, Share, Industry Trends and Forecast to 2033
This report provides insights into the Extracorporeal Membrane Oxygenation (ECMO) Systems market from 2023 to 2033. It includes market size, trends, segmentation, regional analysis, and profiles of key players, offering a comprehensive view of the industry's landscape and future projections.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
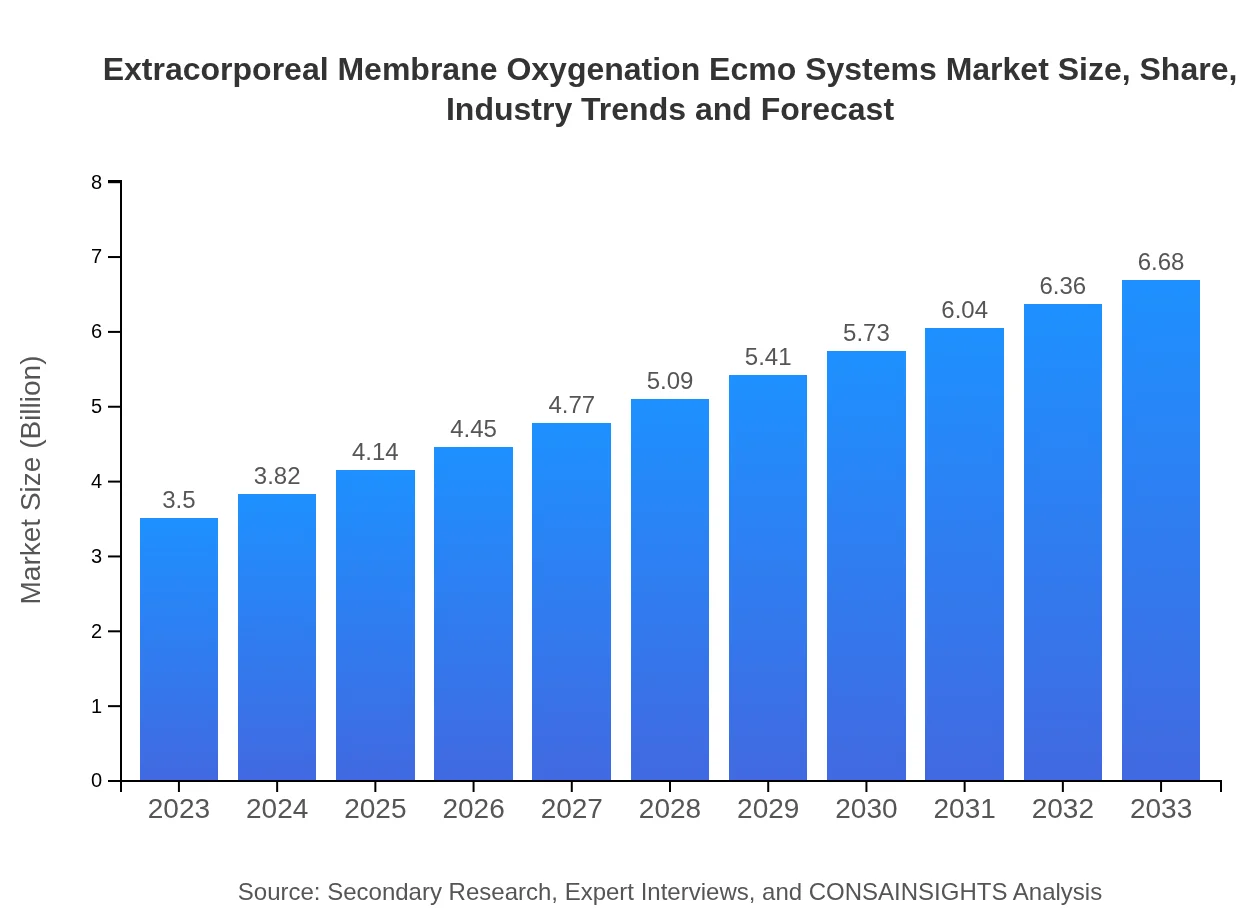
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $6.68 Billion |
| Top Companies | Maquet Cardiopulmonary, Getinge Group, Fresenius SE & Co. KGaA, Medtronic , LivaNova PLC |
| Last Modified Date | 31 January 2026 |
Extracorporeal Membrane Oxygenation Ecmo Systems Market Overview
Customize Extracorporeal Membrane Oxygenation Ecmo Systems Market Report market research report
- ✔ Get in-depth analysis of Extracorporeal Membrane Oxygenation Ecmo Systems market size, growth, and forecasts.
- ✔ Understand Extracorporeal Membrane Oxygenation Ecmo Systems's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Extracorporeal Membrane Oxygenation Ecmo Systems
What is the Market Size & CAGR of Extracorporeal Membrane Oxygenation Ecmo Systems market in 2023?
Extracorporeal Membrane Oxygenation Ecmo Systems Industry Analysis
Extracorporeal Membrane Oxygenation Ecmo Systems Market Segmentation and Scope
Tell us your focus area and get a customized research report.
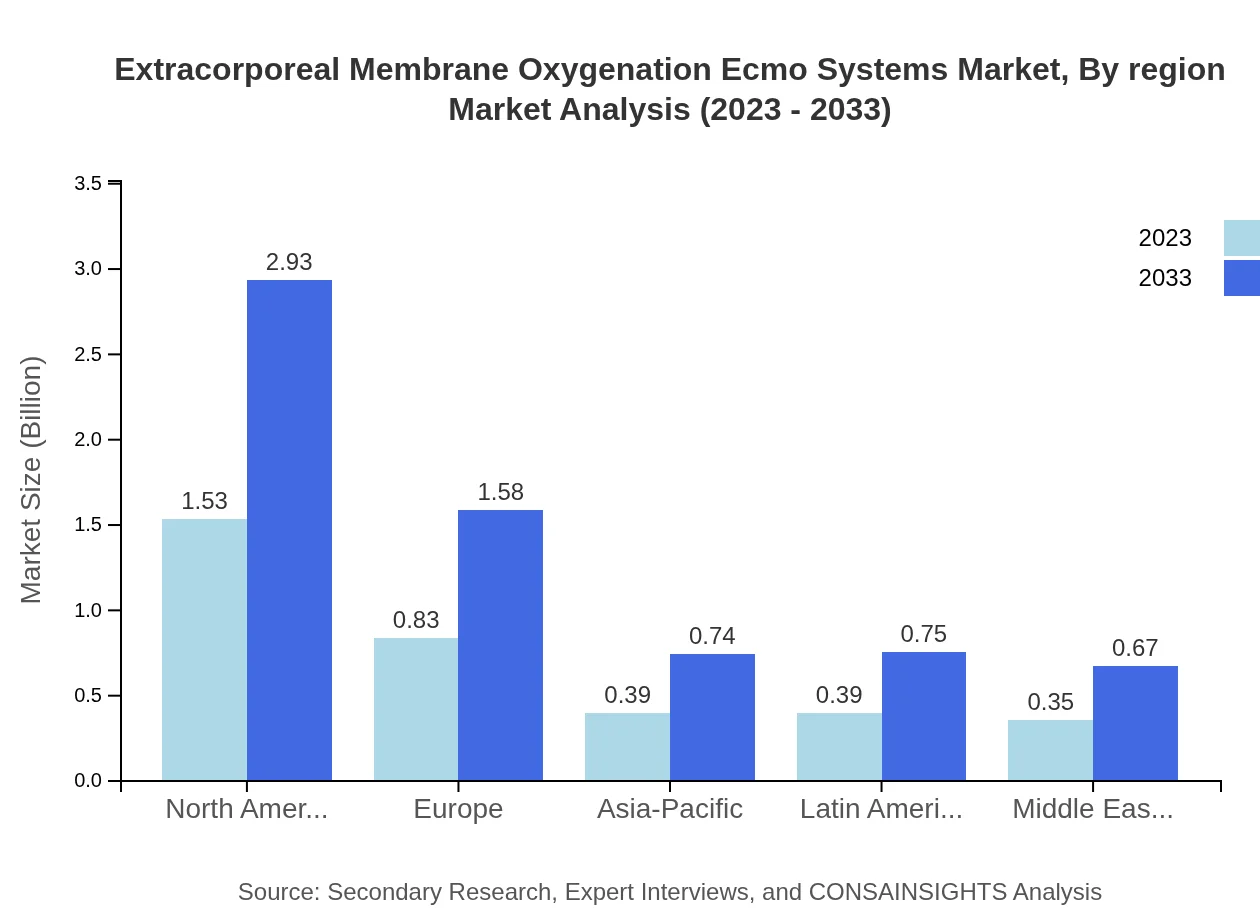
Extracorporeal Membrane Oxygenation Ecmo Systems Market Analysis Report by Region
Europe Extracorporeal Membrane Oxygenation Ecmo Systems Market Report:
Europe is seeing a substantial growth in ECMO systems, forecasted to expand from $1.28 billion in 2023 to $2.45 billion by 2033. This growth is due to increased healthcare budgets, collaboration among medical institutions, and ongoing training initiatives for medical personnel.Asia Pacific Extracorporeal Membrane Oxygenation Ecmo Systems Market Report:
In the Asia Pacific region, the ECMO market is projected to grow from $0.66 billion in 2023 to $1.27 billion by 2033, propelled by increasing healthcare access, improved medical technologies, and growing awareness among healthcare professionals regarding ECMO systems' benefits.North America Extracorporeal Membrane Oxygenation Ecmo Systems Market Report:
North America's ECMO market is expected to rise from $1.13 billion in 2023 to $2.15 billion by 2033, with a significant share attributed to advanced healthcare systems, high awareness, and extensive adoption of ECMO in critical care settings.South America Extracorporeal Membrane Oxygenation Ecmo Systems Market Report:
The South America region faces challenges in the ECMO market, projected to decrease from -$0.03 billion in 2023 to -$0.05 billion by 2033. Limited healthcare infrastructure and lower funding in medical technologies restrain growth despite potential demand.Middle East & Africa Extracorporeal Membrane Oxygenation Ecmo Systems Market Report:
The ECMO market in the Middle East and Africa is also on an upward trajectory, expected to grow from $0.45 billion in 2023 to $0.86 billion by 2033. Increased investments in healthcare facilities and rising incidences of cardiac diseases are primary growth drivers.Tell us your focus area and get a customized research report.
Extracorporeal Membrane Oxygenation Ecmo Systems Market Analysis By Technology
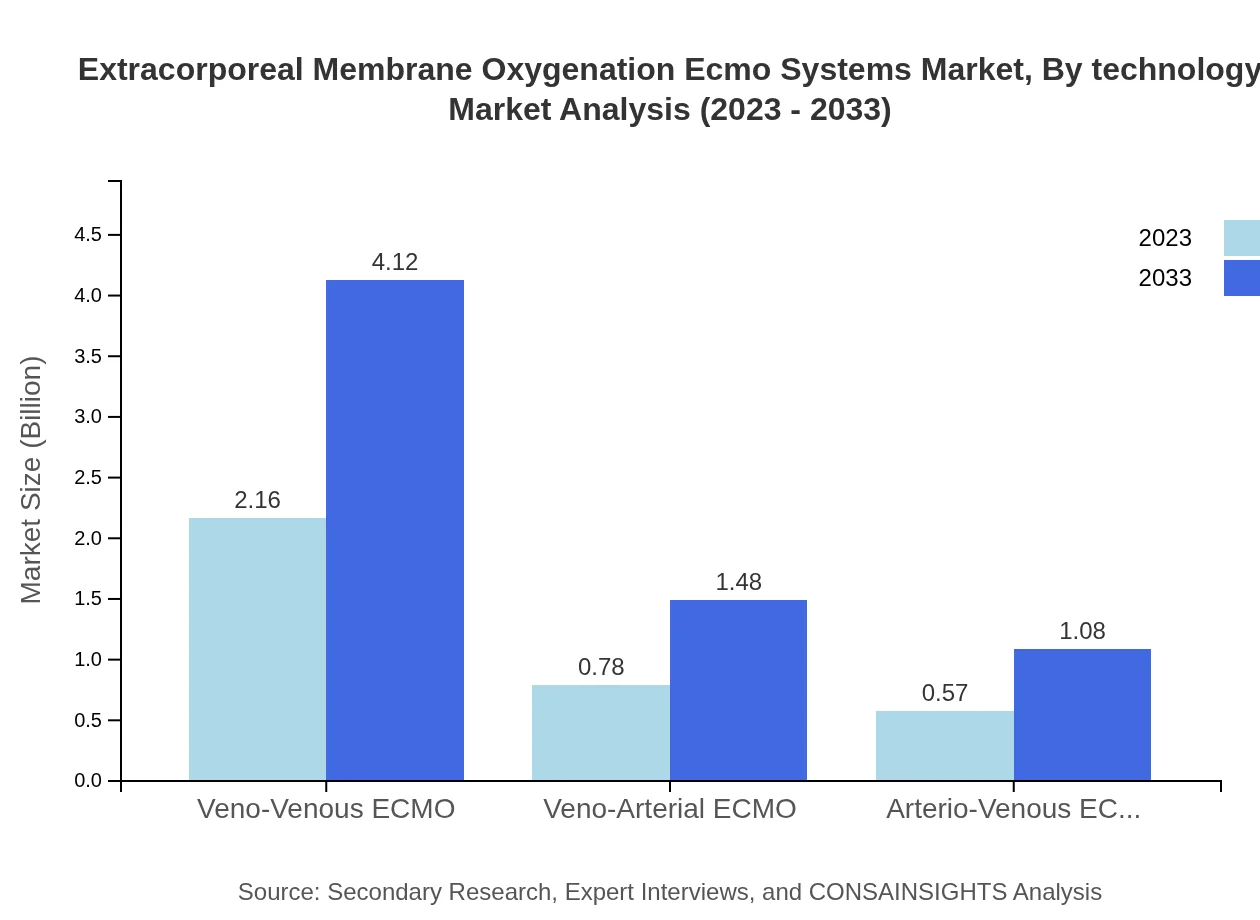
The ECMO market by technology is primarily segmented into veno-venous, veno-arterial, and arterio-venous ECMO systems. Veno-venous ECMO leads this segment, accounting for 61.62% market share in 2023, with a projected growth to 61.62% by 2033. Veno-arterial ECMO holds a 22.23% share, significant for cardiac care, while arterio-venous ECMO, accounting for 16.15%, is utilized less frequently.
Extracorporeal Membrane Oxygenation Ecmo Systems Market Analysis By Application
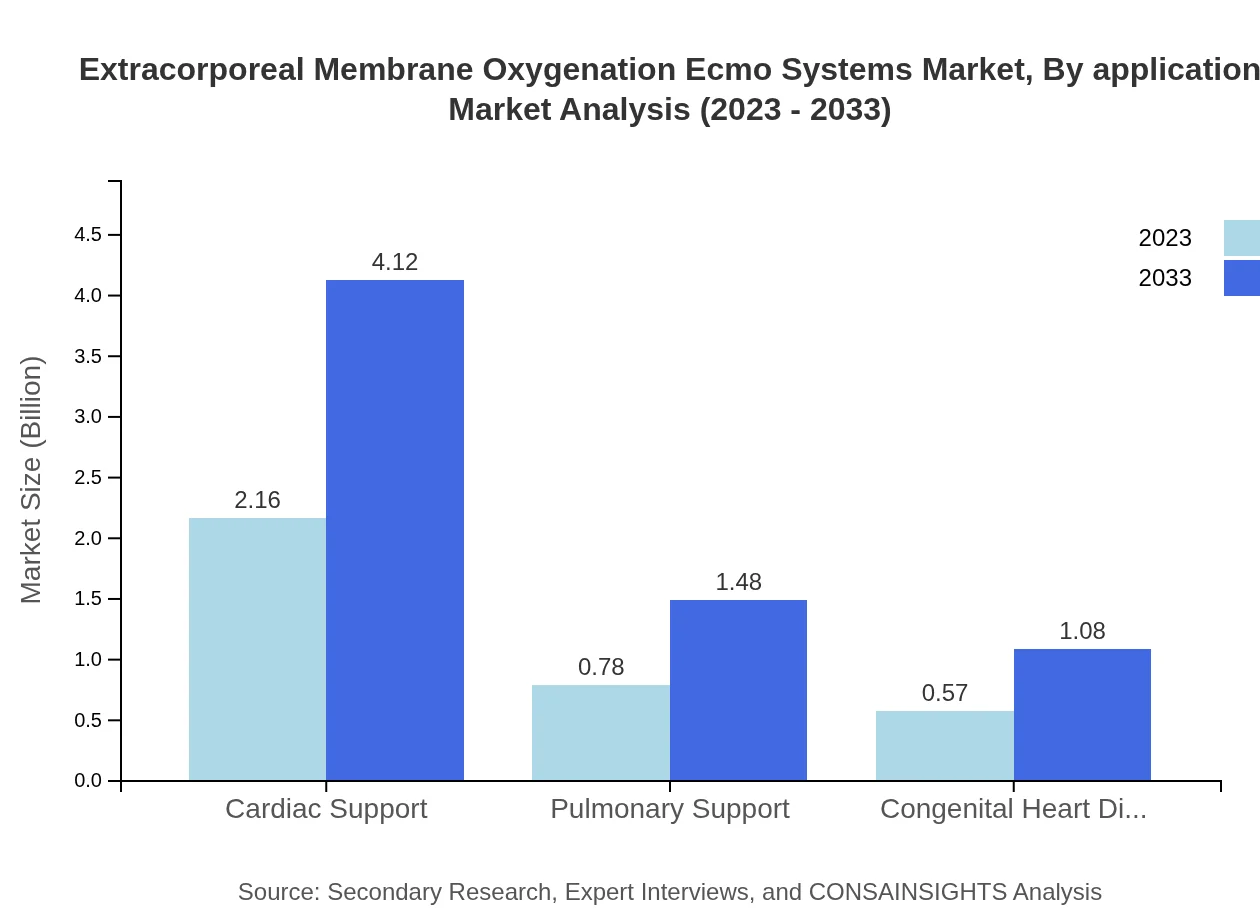
In terms of application, the ECMO systems are used for cardiac and pulmonary support. Cardiac support applications dominate with a 61.62% share in 2023, growing to an equal share in 2033. Pulmonary support follows at 22.23%, also expecting similar stability in the long term, while congenital heart disease cases account for 16.15% of application use.
Extracorporeal Membrane Oxygenation Ecmo Systems Market Analysis By End User
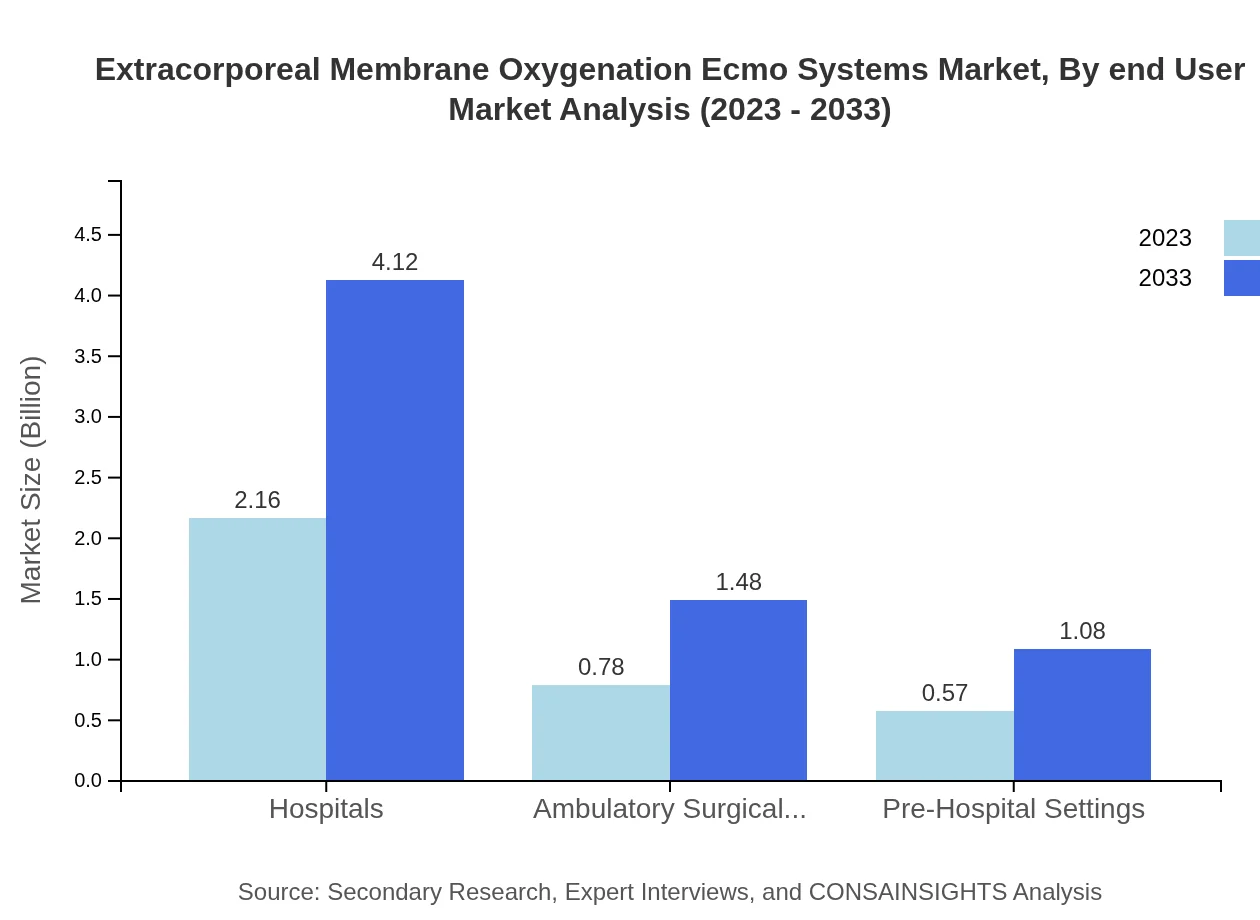
The end-user market is prevalent in hospitals with a notable 61.62% share in 2023, which is expected to hold steady over the forecast duration. Ambulatory surgical centers account for 22.23% and pre-hospital settings at 16.15%, which are both critical in the initial response to medical emergencies.
Extracorporeal Membrane Oxygenation Ecmo Systems Market Analysis By Region
Regional segmentation shows divergent trends with North America leading at 43.84% market share in 2023, expected to remain stable. Europe follows with a 23.69% share, while the Asia-Pacific region captures 11.15%. Latin America and Middle East & Africa hold shares of 11.28% and 10.04%, respectively.
Extracorporeal Membrane Oxygenation Ecmo Systems Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Extracorporeal Membrane Oxygenation Ecmo Systems Industry
Maquet Cardiopulmonary:
A leader in the ECMO market, Maquet offers a comprehensive range of ECMO solutions known for their reliability and advanced technology, contributing significantly to critical care practices globally.Getinge Group:
Known for its innovative medical technology, Getinge Group provides a variety of ECMO devices, enhancing patient outcomes through improved equipment aimed at critically ill patients.Fresenius SE & Co. KGaA:
Fresenius is a prominent manufacturer in the ECMO field, focusing on high-quality and efficient systems designed for use in intensive care environments across the globe.Medtronic :
Medtronic's ECMO systems are pivotal in the market, characterized by their advanced technological features and commitment to patient-centric solutions in acute care.LivaNova PLC:
LivaNova specializes in ECMO technology, providing devices that are critical for both cardiac and respiratory support, positioned as key players within the industry.We're grateful to work with incredible clients.









FAQs
What is the market size of extracorporeal Membrane Oxygenation Ecmo Systems?
The global extracorporeal membrane oxygenation (ECMO) systems market is valued at approximately 3.5 billion USD in 2023, with a projected CAGR of 6.5% anticipated to drive growth through 2033.
What are the key market players or companies in this industry?
Prominent players in the ECMO systems market include Medtronic, MAQUET Cardiopulmonary GmbH, Terumo Corporation, and Sorin Group, which lead the supply of innovative technologies and solutions for critical care management.
What are the primary factors driving the growth in the ECMO industry?
Key growth factors for the ECMO market include increasing incidences of respiratory and cardiac complications, technological advancements in ECMO systems, and enhanced healthcare infrastructure facilitating ECMO usage in critical care.
Which region is the fastest Growing in the ECMO market?
The Asia-Pacific region is the fastest-growing market for ECMO systems, projected to expand from 0.66 billion USD in 2023 to 1.27 billion USD by 2033, reflecting a significant uptick in healthcare investments.
Does ConsaInsights provide customized market report data for the ECMO industry?
Yes, ConsaInsights offers tailored market report data for the ECMO industry, enabling businesses to access specific insights and analytics tailored to their strategic needs and market focus.
What deliverables can I expect from this ECMO market research project?
Expected deliverables include comprehensive market analysis, competitive landscape assessment, trend evaluation, regional insights, and projections over multiple years, all tailored to inform strategic business decisions.
What are the market trends of ECMO systems?
Trends in the ECMO systems market showcase innovations in device functionality, integration of telemedicine, increasing adoption in ambulatory surgical settings, and ongoing advancements in biocompatible materials for improved patient outcomes.