Filgrastim Market Report
Published Date: 31 January 2026 | Report Code: filgrastim
Filgrastim Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Filgrastim market, including insights on market size, trends, segmentation, and forecasts from 2023 to 2033. It aims to guide stakeholders in making informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
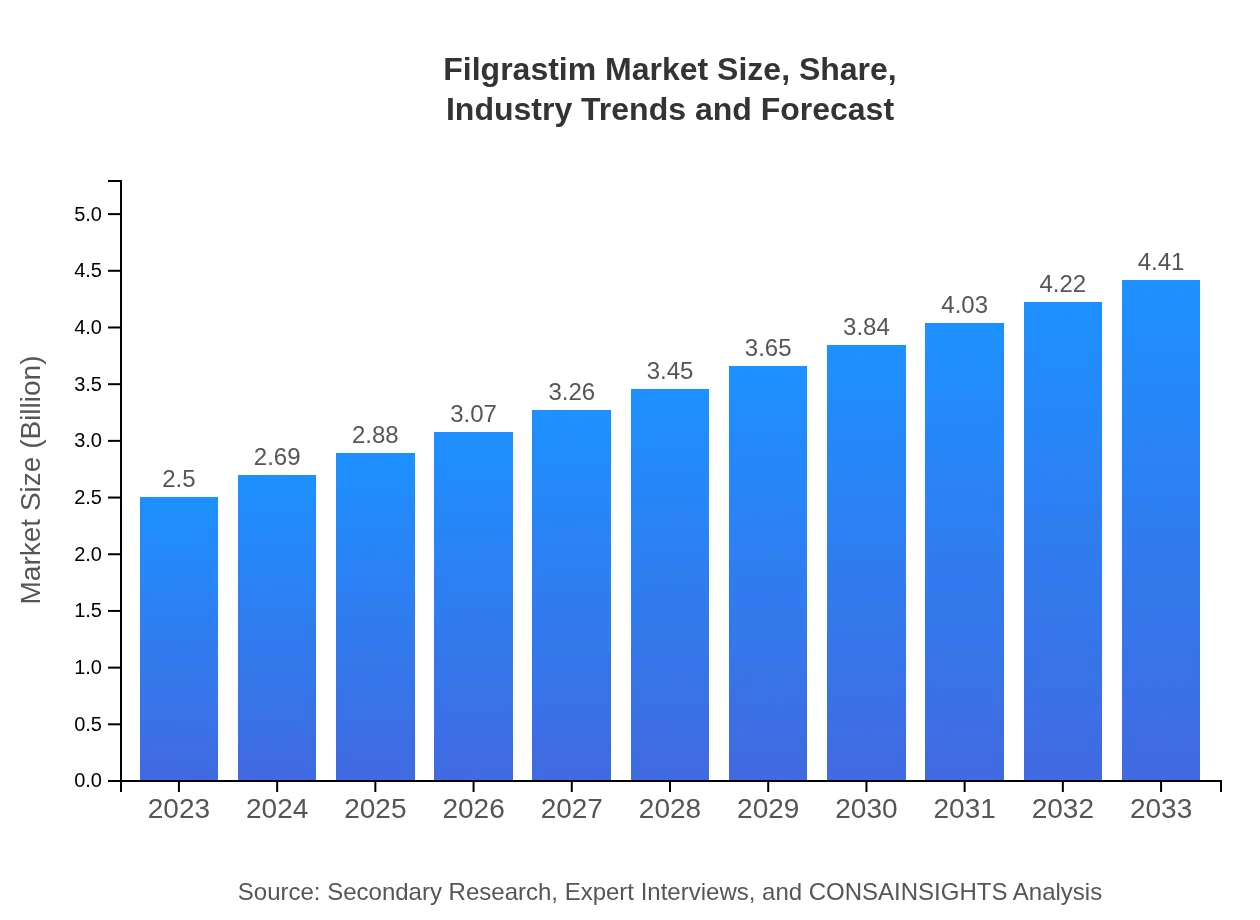
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $4.41 Billion |
| Top Companies | Amgen Inc., Teva Pharmaceutical Industries, Roche, Sandoz |
| Last Modified Date | 31 January 2026 |
Filgrastim Market Overview
Customize Filgrastim Market Report market research report
- ✔ Get in-depth analysis of Filgrastim market size, growth, and forecasts.
- ✔ Understand Filgrastim's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Filgrastim
What is the Market Size & CAGR of Filgrastim market in 2023?
Filgrastim Industry Analysis
Filgrastim Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Filgrastim Market Analysis Report by Region
Europe Filgrastim Market Report:
The European Filgrastim market was valued at $0.77 billion in 2023 and is forecasted to grow to $1.35 billion by 2033. Enhanced research activities and increased collaborations between pharmaceutical firms are driving regional growth.Asia Pacific Filgrastim Market Report:
In the Asia Pacific region, the Filgrastim market was valued at $0.48 billion in 2023 and is expected to grow to $0.84 billion by 2033. The growth is driven by increasing healthcare expenditure and improving access to treatments.North America Filgrastim Market Report:
North America leads the Filgrastim market with a valuation of $0.81 billion in 2023, expected to reach $1.43 billion by 2033. The presence of key market players and advanced healthcare infrastructure contribute to this robust growth.South America Filgrastim Market Report:
The South American Filgrastim market recorded a value of $0.17 billion in 2023, with projections of $0.29 billion by 2033. The rising demand for oncology treatments and supportive care is anticipated to fuel this growth.Middle East & Africa Filgrastim Market Report:
In the Middle East and Africa, the market value stands at $0.28 billion in 2023, with expectations of reaching $0.49 billion by 2033. The growing prevalence of hematological disorders in the region is fostering market expansion.Tell us your focus area and get a customized research report.
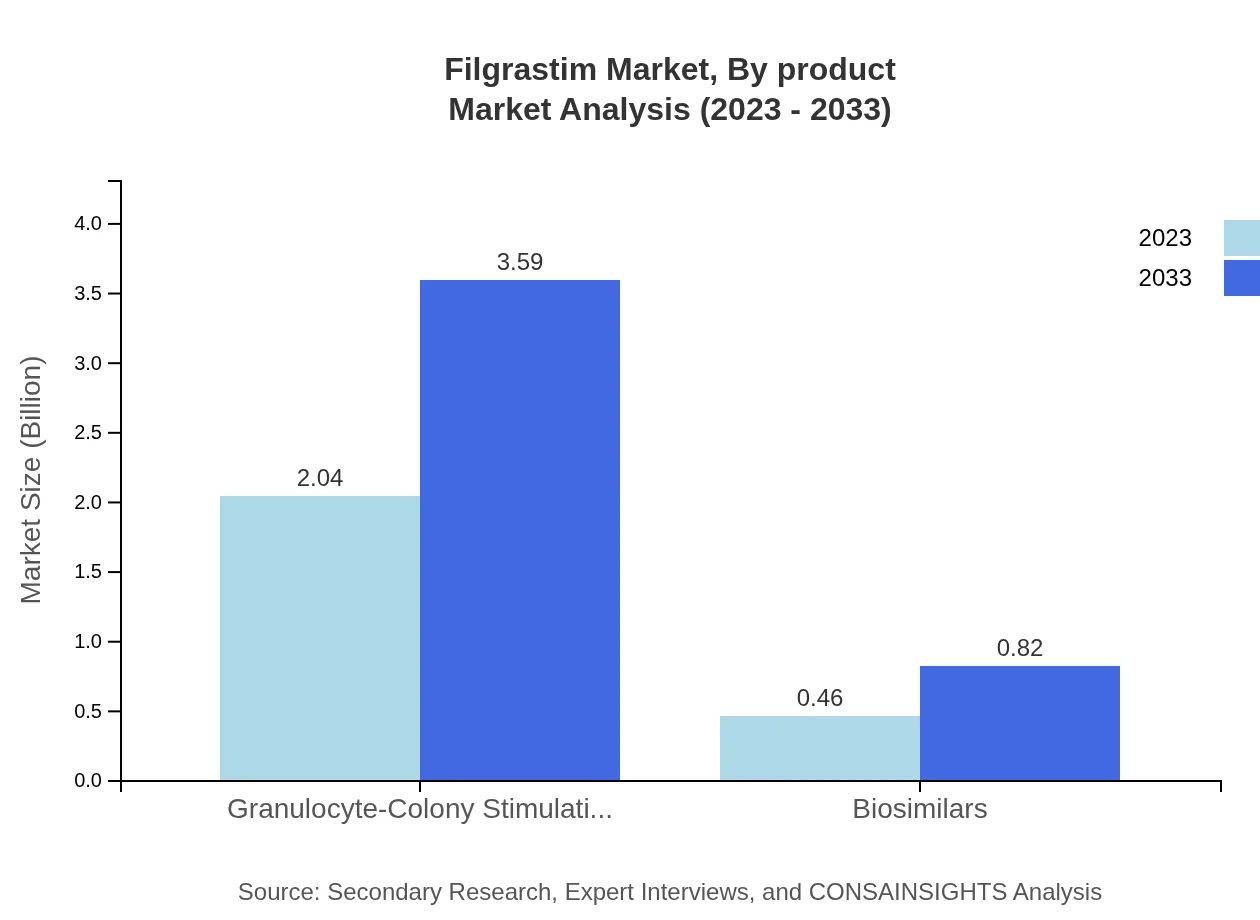
Filgrastim Market Analysis By Product
The Granulocyte-Colony Stimulating Factor (G-CSF) segment dominates the Filgrastim market, with a size of $2.04 billion in 2023, projected to reach $3.59 billion by 2033. Biosimilars provide a competitive edge, boasting a size of $0.46 billion in 2023, forecasted to grow to $0.82 billion by 2033.
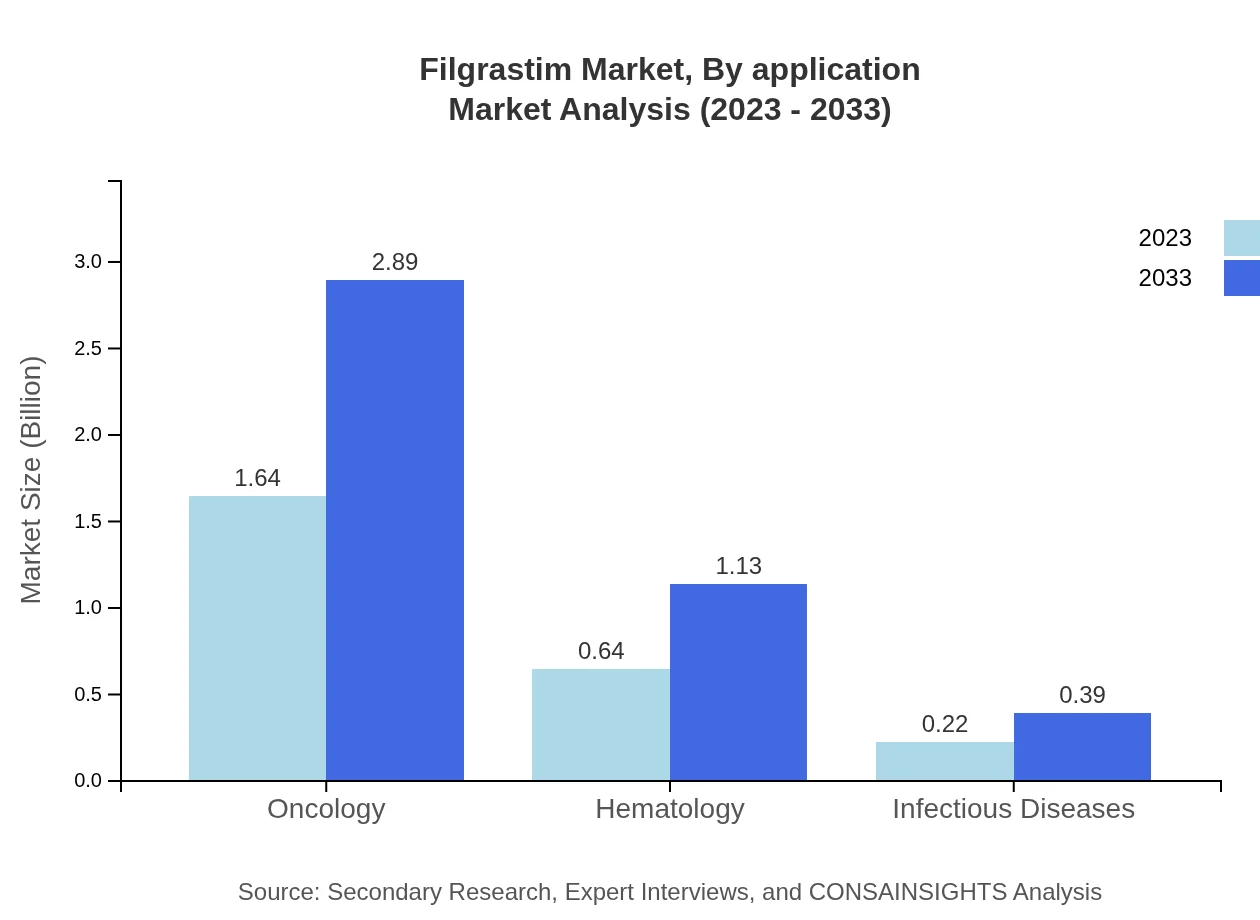
Filgrastim Market Analysis By Application
Applications of Filgrastim primarily include oncology and hematology, with oncology representing the larger share. In 2023, the oncology market size was $1.64 billion, expected to grow to $2.89 billion by 2033. Hematology applications are also expanding with a size of $0.64 billion in 2023, forecasted to reach $1.13 billion by 2033.
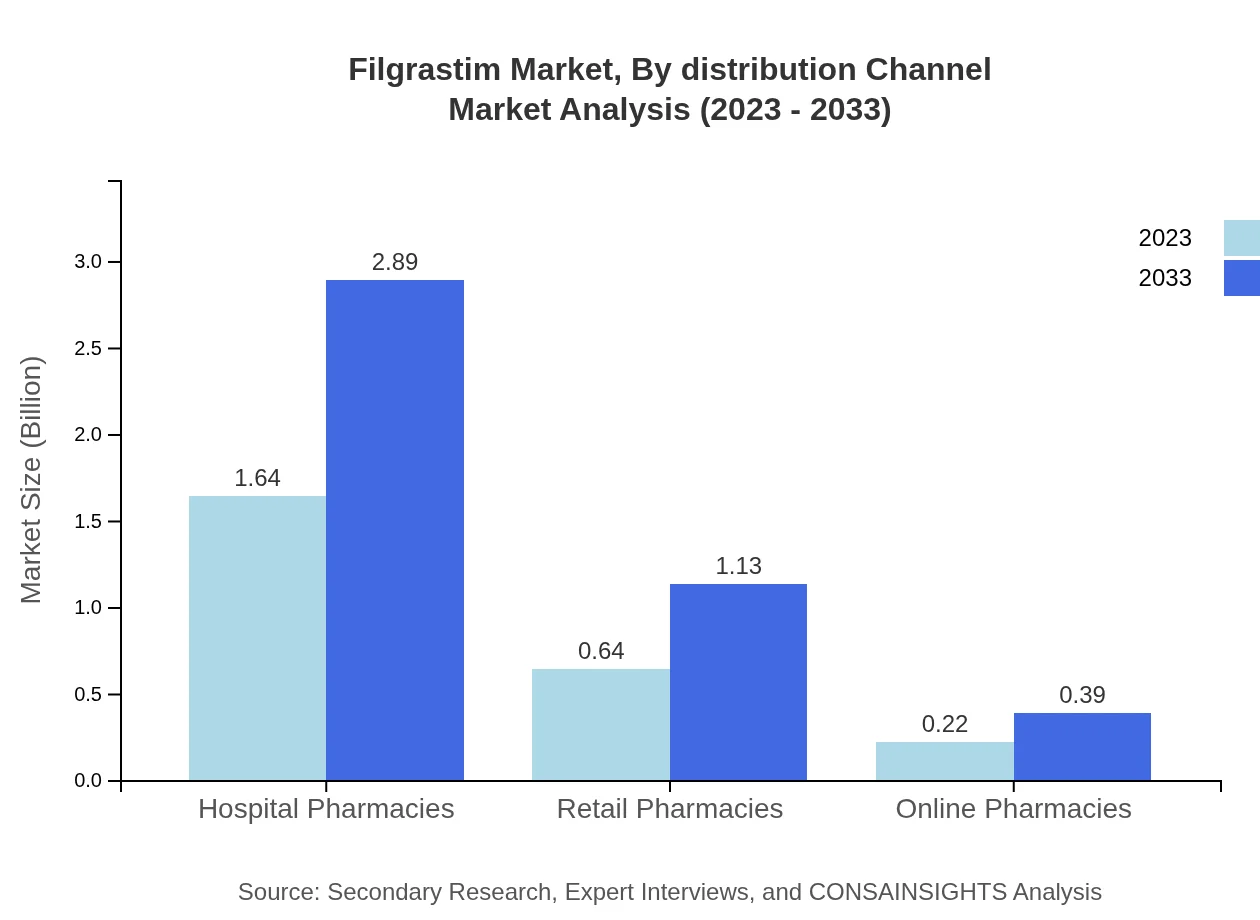
Filgrastim Market Analysis By Distribution Channel
The market is continued to grow across various distribution channels. Hospital pharmacies lead with a 65.61% share, generating a size of $1.64 billion in 2023, projected to increase to $2.89 billion by 2033. Retail and online pharmacies also play crucial roles in widening market access.
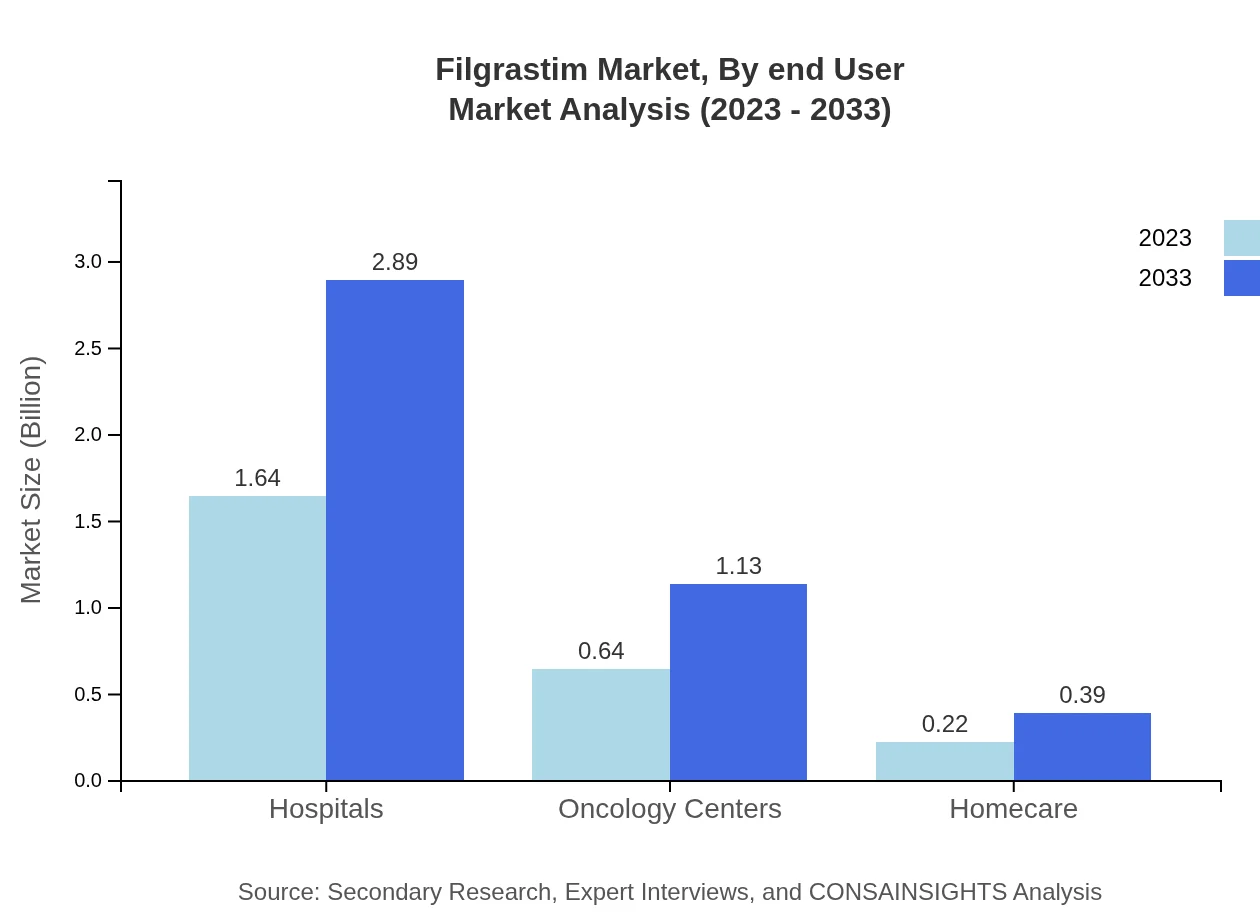
Filgrastim Market Analysis By End User
End-users include hospitals and oncology centers. Hospitals account for $1.64 billion in 2023, with a growth projection to $2.89 billion by 2033. Oncology centers contribute to the growing demand, with market sizes anticipated to rise from $0.64 billion to $1.13 billion by 2033.
Filgrastim Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Filgrastim Industry
Amgen Inc.:
Amgen is a leader in biopharmaceuticals and the original developer of Filgrastim, significantly contributing to its widespread usage and ongoing research.Teva Pharmaceutical Industries:
Teva is a prominent manufacturer of biosimilars, including Filgrastim, delivering affordable and accessible treatments to patients globally.Roche:
Roche engages in continuous innovation in the oncology sector, producing effective treatments that integrate Filgrastim into their protocols.Sandoz:
A division of Novartis, Sandoz specializes in generic medications, including biosimilars of Filgrastim, making healthcare more affordable.We're grateful to work with incredible clients.









FAQs
What is the market size of filgrastim?
The global filgrastim market is projected to reach approximately $2.5 billion by 2033, growing at a CAGR of 5.7%. This robust growth reflects the increasing demand for effective treatments to address neutropenia and other associated conditions.
What are the key market players or companies in this filgrastim industry?
Key players in the filgrastim market include major pharmaceutical companies specializing in biotechnology and oncology treatments. Leading firms actively engaged include Amgen, Novartis, and Teva, which significantly contribute to the market through innovative products and distribution strategies.
What are the primary factors driving the growth in the filgrastim industry?
Growth in the filgrastim market is primarily driven by the increasing incidence of cancer, a rising geriatric population, and advancements in drug formulations. Additionally, the expanding applications of filgrastim in clinical settings contribute to its market potential.
Which region is the fastest Growing in the filgrastim?
The fastest-growing region in the filgrastim market is North America, projected to grow from $0.81 billion in 2023 to $1.43 billion by 2033. Strong healthcare infrastructure and rising cancer prevalence drive this growth in the region.
Does ConsaInsights provide customized market report data for the filgrastim industry?
Yes, ConsaInsights offers customized market report data for the filgrastim industry. Clients can request tailored insights, including specific market segmentation, regional analysis, and competitive assessments to meet unique business needs.
What deliverables can I expect from this filgrastim market research project?
Deliverables from a filgrastim market research project typically include a comprehensive report detailing market size, growth forecasts, competitive landscape analysis, regional insights, and strategic recommendations to aid business decision-making.
What are the market trends of filgrastim?
Current market trends in the filgrastim industry include increased adoption of biosimilars, expanding global access to therapies, and innovative treatment protocols in oncology and hematology, reflecting the shifting landscape towards more targeted and personalized medicine.